Figure 1: Core M3 α-DG glycosylation process and ECM binding.
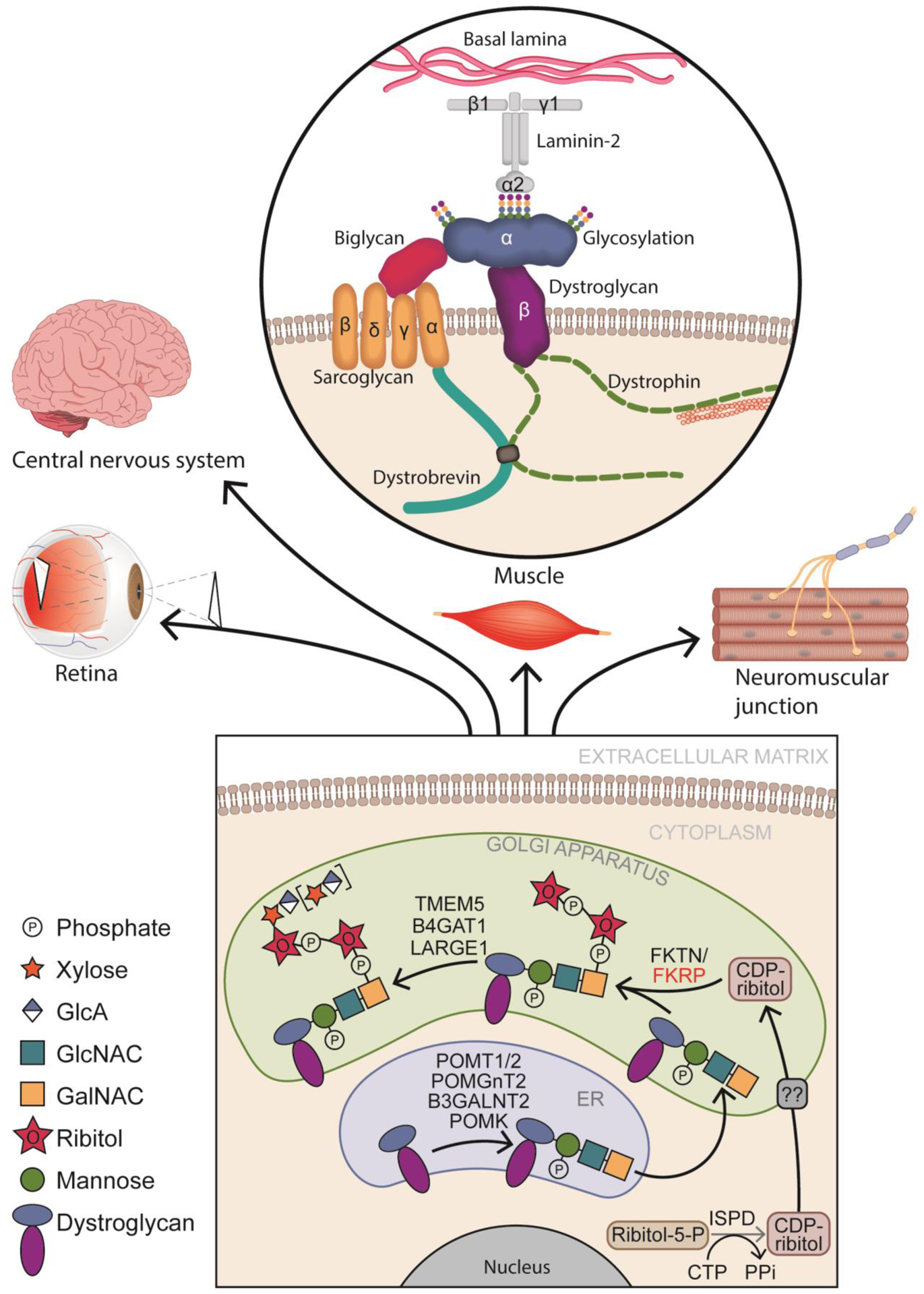
In the endoplasmic reticulum (ER), the glycosylation process begins when protein O-mannosyl transferase 1/2 (POMT1/2) complex catalyzes the addition of a mannose group to serine/threonine residues on α-dystroglycan (DG). Subsequently N-acetylglucosaminyltransferase 2 (POMGNT2) and β1,3-N-acetylgalactosaminyltransferase 2 (B3GALNT2) attach sequentially a N-acetylglucosamine (GlcNAc) and a N-acetylgalactosamine (GalNAc). Protein-O-mannose kinase (POMK) phosphorylates mannose. Once in the golgi apparatus, fukutin (FKTN) and fukutin related protein (FKRP) will use cytidine 5-diphosphate (CDP)-ribitol produced by isoprenoid synthase domain-containing protein (ISPD), to transfer tandem ribitol-5-phosphate onto the 3-GalNAc-β3-GlcNAc-β4-(6-P)-Man-O-(Ser/Thr) modification on α-DG. Afterwards, transmembrane protein 5 (TMEM5) and b-1,4-glucuronyltransferase 1 (B4GAT1) add a β1,2-Xylose (Xyl) and a β1,4 glucuronic acid (GlcA) allowing like-acetylglucosaminyltransferase 1 (LARGE1) to add α1,3-Xyl-β1,3-GlcA repeats. Glycosylated α-DG will bind to laminin α−2 in the muscle sarcolemma, as well as to other components of the extracellular matrix (ECM) in the retina, the central nervous system and at the neuromuscular junction.
