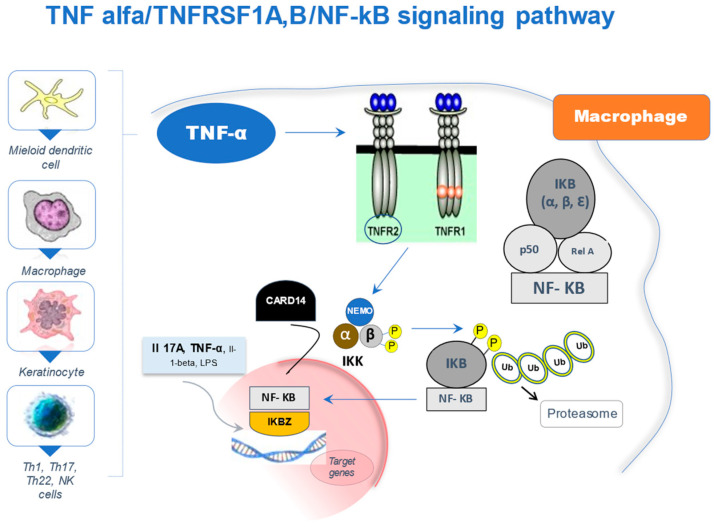
Figure 1.
The TNF-α/TNFRSF1A,B/NF-KB signaling pathway. TNF-α is a pro-inflammatory cytokine synthesized by various cell lineages, mainly macrophages, dendritic cells, keratinocytes as well as various T cell types. It exerts its functions through binding to type 1 (TNFR1) and 2 (TNFR2) cell membrane receptors. TNFR2 (TNFRSF1B) shows higher affinity for TNF-α. NF-κB is a dimer made up of Rel family proteins (p50, p52, Rel A/p65, cRel, and Rel B), the p50/p65 heterodimer being the most common. In the absence of TNF-α stimulation, the activity of NF-κB is regulated at cytoplasmic level by an inhibitory complex of proteins called IKB or Kappa Beta inhibitors (IkB alpha, beta, and epsilon) that regulate its function by blocking its translocation to the nucleus. The binding of TNF-α to its receptors triggers the activation of the inhibitor kinases of NF-κB or “IKK complex” consisting of IKK-alpha, IKK-beta and IKK-gamma or NEMO. The characteristic event is the phosphorylation of IKB-alpha by the IKKa/b complex, inducing its ubiquitination and degradation by the proteasome; and thus, allowing the release of NF-κB. Once in the nucleus, NF-κB meets another inhibitory protein called IKBZ. Likewise, IKBZ can act as a transcription factor regulated by IL-17A, IL-1beta, and to a lesser extent, by TNF-α. In turn, IKBZ plays an important role in the development and expansion of Th17 lineages. Finally, CARD14 activates NF-κB by partially known mechanisms (see text for more details), although some authors suggest that CARD14 would activate the IKK complex, consequently regulating the activity of NF-κB, leading to an increase in its transcriptional activity. TNF-α: tumor necrosis factor-alpha. TNFR1 (TNFRSF1A): TNF-α type 1 receptor. TNFR2 (TNFRSF1B): TNF-α type 2 receptor. NF-κB: nuclear factor of kappa light chain enhancer of activated B cells. IKB: kappa-beta inhibitors. IKK: inhibitor kinases of NF-κB. IKBZ: z-inhibitor protein of NF-κB. IL: interleukin. Th: T-helper. CARD14: caspase recruitment domain family member 14.