Figure 1.
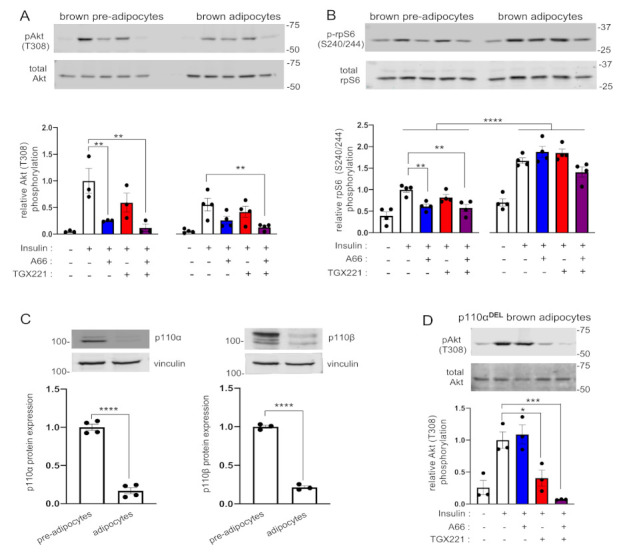
p110α is the principal PI3K isoform engaged in insulin signalling in brown adipocytes. Brown pre-adipocytes or differentiated adipocytes were treated with A66 (1 μM, blue graph bars) or TGX221 (0.5 μM, red graph bars) or a combination of both inhibitors (purple graph bars) followed by stimulation with 100 nM of insulin for 15 min at 37 °C. (A) Levels of Akt (T308) phosphorylation were determined by immunoblot analysis. Phosphorylation levels were normalised to total Akt levels detected in a second blot performed in parallel using the same lysates. (B) Ribosomal protein S6 (rpS6) (S240/244) phosphorylation was also detected on the same immunoblots. (C) The levels of expression of p110α and p110β in pre-adipocytes and differentiated adipocytes used in the signalling experiments were also determined by immunoblot analysis. p110α and p110β signal intensities were normalised to vinculin (used as a loading control). (D) Akt (T308) phosphorylation in p110α-deficient (p110αDEL) brown adipocytes treated and stimulated as above. Representative immunoblots and bar graphs with data from three or four (n = 3–4) independent experiments are shown. Data are presented as mean ± SEM. Statistical analysis was performed by one-way ANOVA with Dunnett’s multiple comparisons test between insulin-stimulated vehicle-treated and insulin-stimulated inhibitor-treated samples (A,B,D) or by unpaired two-tailed t-test (C). Comparisons between pre-adipocytes and differentiated adipocytes in (B) were performed by two-way ANOVA with Sidak’s multiple comparisons test. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
