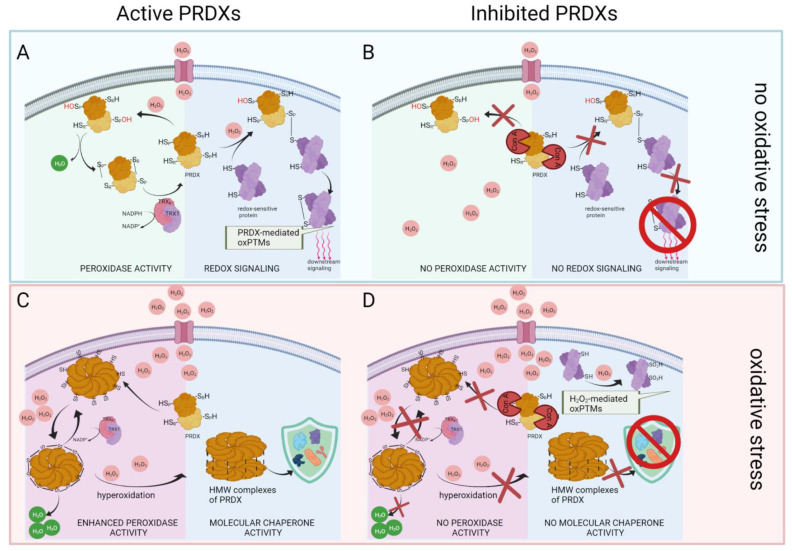
Figure 8.
Theoretical image of the effects of inhibition of PRDX activity under non-stress and oxidative stress conditions. In non-stress conditions, active 2-Cys PRDX scavenges H2O2 through the formation of a sulfenic acid (Cys-SOH) on the peroxidatic cysteine (CysP), with the subsequent formation of a disulfide bond between Cysp and the resolving cysteine (CysR) [32]. Oxidative equivalents can be transferred from PRDX to redox-sensitive target proteins, thus enabling re-dox-signaling [13] (A). Covalent binding of Con A to the peroxidatic cysteines of 2-Cys PRDXs results in the inhibition of peroxidative and redox relay activity [24] (B). Under oxidative stress conditions, an equilibrium exists between the dimer and decamer structures, with the reduced (SH) decamer being the most efficient peroxidase [13]. The decamers can further undergo overoxidation and form high-molecular-weight (HMW) oligomers exhibiting molecular chaperone functions [14] (C). Inhibition of 2-Cys PRDX under oxidative stress conditions results in lack of both the peroxidative function of PRDX and the molecular chaperone function (D).