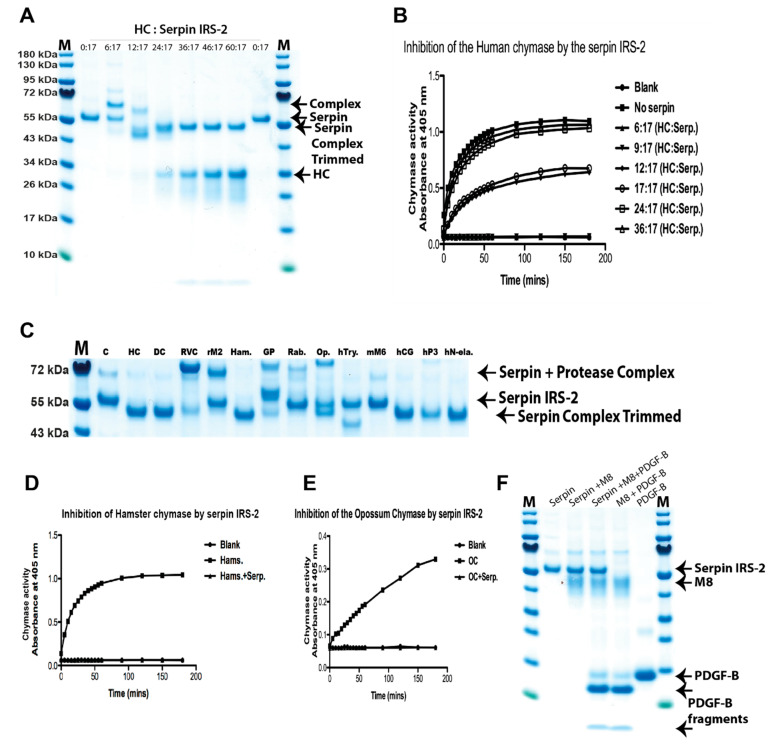
Figure 3.
Inhibiting activity of the tick serpin IRS-2 on a panel of mammalian hematopoietic serine proteases. Panel (A) shows a titration of the activity of tick IRS-2 on human mast cell chymase. The amount of the recombinant tick IRS-2 was constant in all lanes. The amount of human chymase increased from left to right, starting and ending with two lanes with no chymase to show the size and purity of the recombinant IRS-2 and a reference point to evaluate the different complexes and cleavage products that appear following the interaction between the serpin and the enzyme. The ratio is then presented as follows. No chymase and 17 molar equivalents IRS-2 (0:17), six molar equivalents chymase and 17 equivalents IRS-2 (6:17) and until (60:17). The covalent serpin-chymase complex, the serpin, the trimmed serpin chymase complex and the unbound chymase are all marked by arrows to the right side of the panel (A). Panel (B) shows a chromogenic substrate assay of the different samples shown in Figure 3A using a chromogenic substrate (Suc-AAPF-pNA) for chymotrypsin-like proteases. In a ratio where the remaining free chymase starts to appear in panel (A) (12:17), cleavage of the substrate is also starting. Panel (C) shows an analysis of the inhibiting activity of a panel of hematopoietic serine proteases ranging from human to platypus enzymes, the same as in Figure 2, except mMCP-8, which is analyzed in panel (F). All proteases in this panel are inhibited by the serpin except for the rabbit Leu-ase and the tryptases, human tryptase and mMCP-6. The covalent serpin-enzyme complex, the serpin and the trimmed serpin protease complex are marked by arrows to the right side of the panel (C). Panels (D,E) show the activity of the serpin on hamster and opossum chymases using a chromogenic substrate. The panels also indicate that the trimmed complexes, seen in panel (C), are enzymatically inactive due to the serpin that is covalently bound to the active site of the enzymes. Panel F shows the potential interaction between the serpin and mMCP-8. mMCP-8 is not inhibited by IRS-2 and can cleave the substrate mouse PDGF-B even in the presence of the serpin. The serpin, mMCP-8, uncleaved mouse PDGF-B, and the two fragments of mouse PDGF-B generated after cleavage by mMCP-8 are all marked by arrows to the right side of the panel (F).