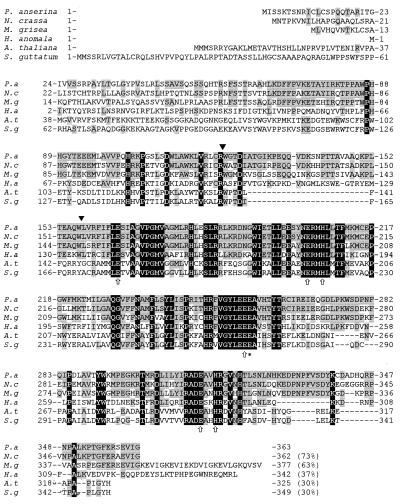
FIG. 3.
Multiple protein alignment of the deduced amino acid sequences of P. anserina PaAOX with N. crassa AOD-1 (29), M. grisea MgAOX (61), Hansenula anomala (49), Arabidopsis thaliana AOX1B (48), and S. guttatum AOX1 (46). The area with black background corresponds to residues completely conserved between all species, while the area with gray background displays homology of PaAOX with the AOX sequence of several but not all examples (indicated in percent at the end of the sequence). The amino acids indicated by arrows were proposed to form the binuclear iron center on the matrix side of the mitochondrial inner membrane, whereas Glu270 (∗) was found to be essential for the catalytic activity of the alternative oxidase from S. guttatum (1, 4, 53). These amino acids are also conserved in PaAOX. The positions of two introns in the nucleotide sequence of PaAox are indicated by black triangles.