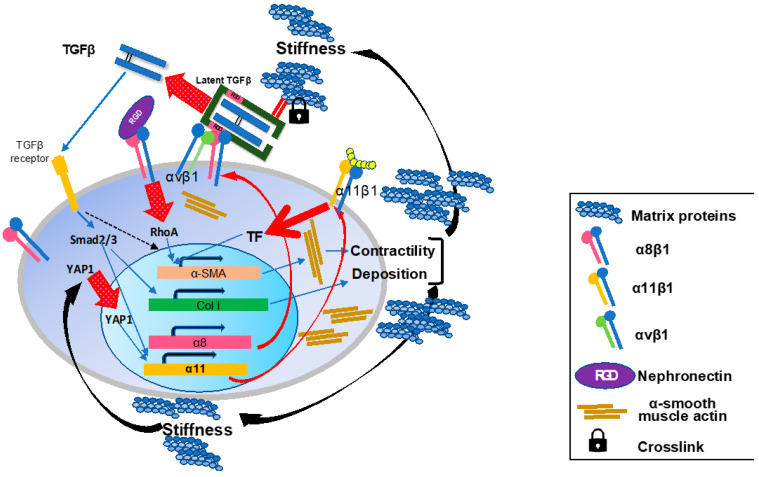
Figure 8.
Schematic summary of the roles of the integrins on activated hepatic stellate cells in liver fibrosis. During the fibrogenesis, α8 and α11 subunits are induced on activated HSCs/myofibroblasts. In the fibrotic milieu of the tissue, increased matrix rigidity induces intranuclear translocation of YAP1 and initiates transcription of Itga11 gene. Induced a8b1 and α11β1 both promote a-SMA expression, actin fiber formation, and cellular contractility upon ligand-engagement with such as nephronectin and collagen type I, respectively. Once HSCs acquire the myofibroblast phenotype, the cells contribute more to TGFb activation on their surface through interactions of integrins including a8b1 and αvβ1 with pro-TGFb in the surrounding matrix proteins. Released matured TGFb binds to its receptor and initiates the Smad signaling cascade. The signal promotes production of matrix proteins containing collagen species from myofibroblasts and α11 expression. Crosstalk between TGFβ-initiated and a8b1- and α11β1-mediated signals cooperatively enhance a-SMA expression. These effects by a8b1 and α11β1 on HSCs consequently render myofibroblasts highly contractile and productive for collagens and other matrix proteins, which reinforces tissue stiffness to maintain and enhance a11 expression.