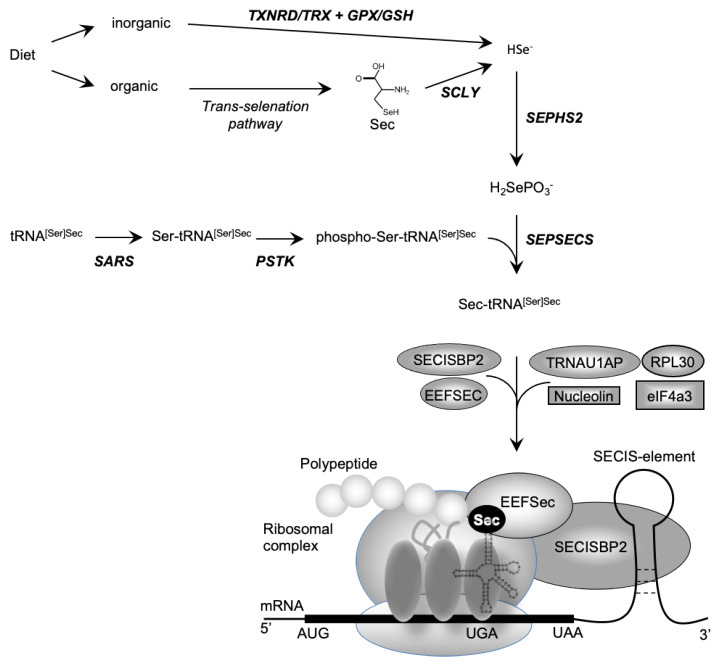
Figure 1.
Biosynthesis of selenocysteine (Sec) and selenoproteins. Dietary sources of selenium exist in inorganic form (e.g., selenate, selenite) and organic form (e.g., Sec, SeMet). Inorganic selenium is reduced to selenide by TXNRD/TRX or GPX/GSH systems and organic selenium is metabolized to Sec, used by SCLY to generate selenide. De novo Sec synthesis takes place on its own tRNA (tRNA[Ser]Sec), which undergoes maturation through sequential modifications (SARS-mediated addition of Ser, PSTK-mediated phosphorylation of Ser), with acceptance of a selenophosphate (generated from selenide by SEPHS2) catalysed by SEPSECS as final step. Expression of selenoproteins requires recoding of an UGA codon as the amino acid Sec instead of a premature stop. The incorporation of Sec is mediated by a multiprotein complex containing SECISBP2, bound to the SECIS element situated in the 3′-untranslated region of selenoproteins, the Sec elongation factor EEFSEC, together with Sec-tRNA[Ser]Sec at the ribosomal acceptor site. The other factors (e.g., ribosomal protein L30, eukaryotic initiation factor eIF4a3, nucleolin) have regulatory roles.