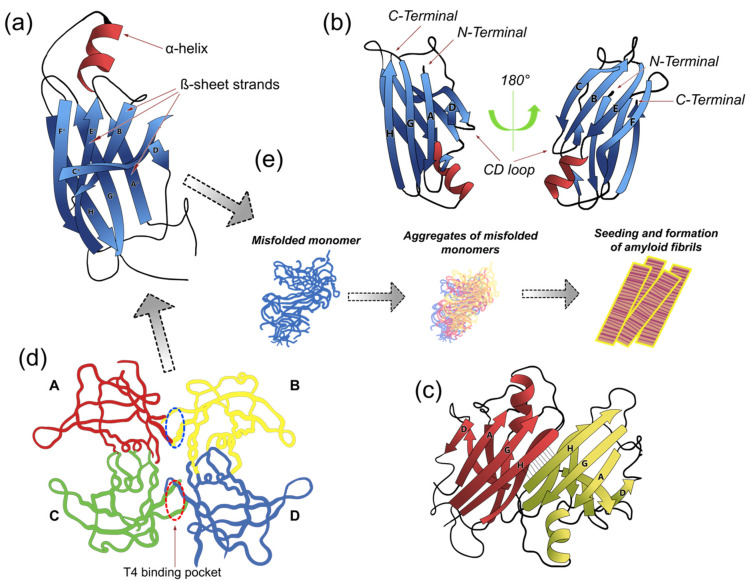
Figure 1.
Transthyretin (TTR) conformational structures. TTR is a homotetrameric protein composed of four monomers of 127 amino acids each. Each monomer contains one small α-helix and eight β-strands (CBEF and DAGH) (a,b), which are arranged in a β-sandwich of two four-stranded β-sheets and one small α-helix found between β-strands E and F. TTR monomers interact via hydrogen bonds between the antiparallel, adjacent β-strands H-H’ and F-F’ to form a dimeric species (c). The two dimers (A-B and C-D) form the tetramer through hydrophobic contacts between the residues of the A and B, and G and H loops. The tetramer forms a central hydrophobic pocket (T4 channel) with two binding sites for hormones (red and blue ovals in (d)). (e) The TTR tetramer dissociates into dimers and lowest free-energy monomers more prone to form fibrils; mutant monomers misfold, aggregate, and subsequently form prefibrillar compounds and amyloid fibrils. In this scenario, tetramer dissociation into monomers is the rate-liming step of the aggregation reaction. Based on this model, several studies have focused on developing effective and selective therapeutic approaches (i.e., TTR ligands) aimed to prevent TTR dissociation and aggregation. (Model adapted from PDB code 1DVQ (https://pdbjbk1.pdbj.org/emnavi/quick.php?id=pdb-1dvq, (accessed on 5 October 2021)).