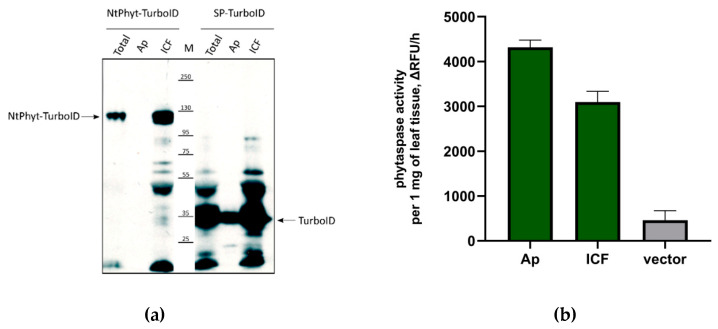
Figure 2.
Evaluation of the stability of NtPhyt-TurboID and SP-TurboID proteins in the apoplast and inside the plant cells. (a) For NtPhyt-TurboID- and SP-TurboID-producing leaves, proteins in the apoplastic washes (Ap) and intracellular fractions (ICFs) were analysed by Western blotting with HisProbe detection. ‘Total’ represents leaf extracts without fractionation. Equal amounts of leaf tissues (5 mg) were taken for protein analyses, and equivalent 15 μL aliquots of the subcellular fractions were loaded on the gel. M, molecular weights of protein markers. Arrows indicate positions of the recombinant proteins. (b) Measurement of phytaspase proteolytic activity in the extracellular (Ap) and intracellular (ICF) fractions obtained from NtPhyt-TurboID-producing leaves. ‘Vector’ total protein sample from leaves infiltrated with agrobacteria carrying the empty vector. Ac-VEID-AFC (20 μM) was used as the phytaspase substrate for quantitative assessment of phytaspase proteolytic activity. Relative rates of hydrolysis were determined as an increase of relative fluorescence units per hour (deltaRFU/h). Enzymatic activities were normalized by the weight of leaf tissues taken for analysis. Data represent the mean of three independent experiments ± standard deviation (SD).