Figure 2. Global and cardiac-specific loss of PHD2/3 impact cardiac metabolism.
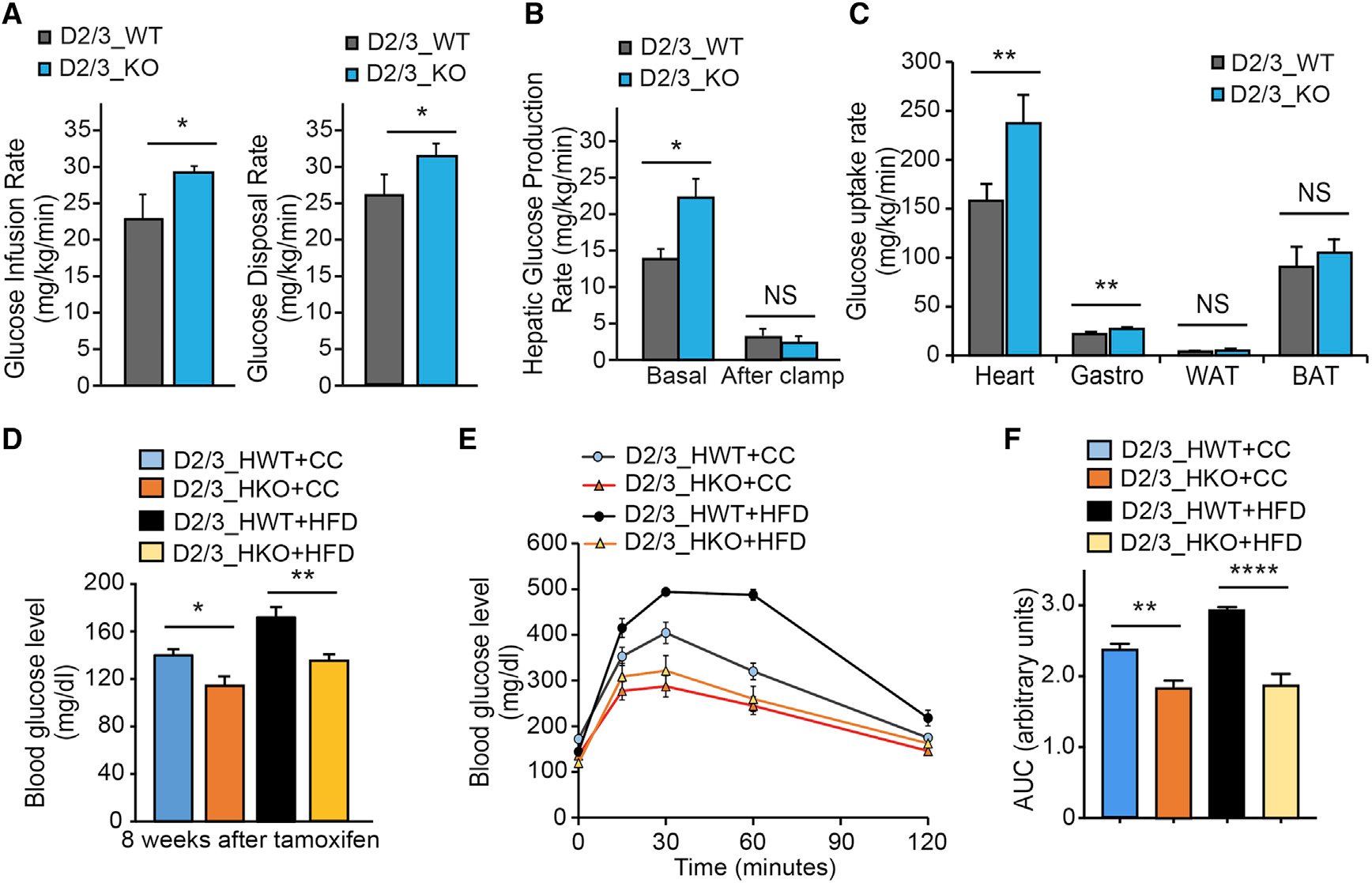
(A–C) Hyperinsulinemic-euglycemic clamp in conscious D2/3_KO and D2/3_WT mice at 4 weeks after tamoxifen injection (n = 6 per each group). Glucose metabolism is significantly increased in D2/3_KO mice (A). Hepatic glucose production rate is shown in (B). Tissue glucose uptake is shown in (C), showing a predominant increase of glucose uptake in the heart of D2/D3_KO mice, and also in Gastrocnemius muscle at a lower range (C).
(D–F) Cardiomyocyte-specific disruption of PHD2/3 was triggered by tamoxifen injection (20 mg/kg/day) for 4 consecutive days in D2/3_HKO mice. Mice from both genotypes were split in two groups randomly and fed with normal chow (CC) or high-fat diet (HFD) for 8–12 weeks (n = 6–11 mice per group). The glucose blood level was monitored at the 8-week time point following PHD2/3 disruption (D). D2/3_HKO mice showed a significantly lower blood glucose level, at both CC and HFD-feeding conditions. (E) Glucose tolerance test at 8 weeks following CC or HFD in D23_HKO and D2/3_HWT mice. Similarly, as expected, HFD-D2/3_HWT mice had a pre-diabetic-specific slower response to glucose, whereas D2/3_HKO mice exhibited a faster response to glucose, which remained equivalent regardless CC or HFD feeding. (F) Area under the curve from data shown in (E), depicting significant difference among the groups of mice.
Data are shown as averages ± SEM. Statistically significant differences are indicated by asterisks: *p < 0.05, **p < 0.01, and ***p < 0.001.
