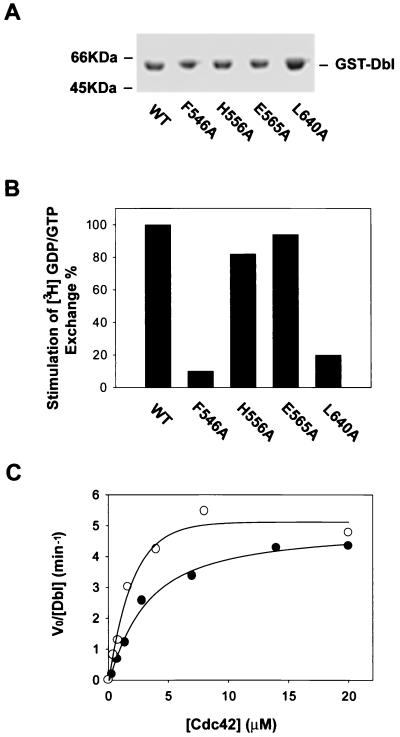
FIG. 4.
Effect of CR2 mutations on the GEF activity of onco-Dbl. The cDNAs encoding the wild-type DH-PH module or the module bearing mutations in the DH domain were cloned into an insect cell transfer vector with an N-terminal GST fusion tag for functional expression in Sf9 insect cells. (A) Coomassie blue-stained SDS-PAGE gel of the insect cell-expressed, glutathione-agarose affinity-purified GST fusion mutants. WT, wild type. (B) Relative GEF activities of the recombinant DH domain mutants on RhoA. Approximately 0.2 μg of purified GST-Dbl or Dbl mutant was incubated with 1 μg of [3H]GDP-loaded RhoA in the GEF reaction buffer for 5 min before termination of the reaction by nitrocellulose filtration. The percent retention of RhoA-bound [3H]GDP catalyzed by the mutants was normalized to that catalyzed by wild-type Dbl. (C) Derivation of the kinetic parameters of wild-type Dbl and the H556A mutant using Cdc42 as substrate. The V0s were determined in the presence of 20 nM Dbl or H556A mutant at 1-min intervals with various concentrations of Cdc42-GDP. The resulting V0 and substrate concentration data were best fitted into a modified Michaelis-Menton equation, with corrections being made for basal GDP dissociation from Cdc42.