Table 4.
Examples of using the click chemistry reaction to obtain new alginate derivatives.
| Click Reaction | Functional Groups Involved in Reaction | Characteristic |
|---|---|---|
| Copper-(I)-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) |
Azide–Alkyne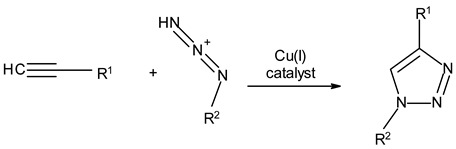
|
Cu-catalyzed (cytotoxic and difficult to remove from product) Reversible Bioorthogonal No side product |
| Strain-Promoted Alkyne-Azide Cycloaddition (SPAAC) |
Azide–cyclic Alkyne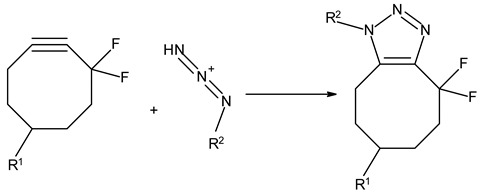
|
No catalyst needed Hydrophobicity of alkyne ring hindering the reaction |
| Inverse Electron Demand Diels–Alder Cycloaddition (IEDDA) | Dienophile–Diene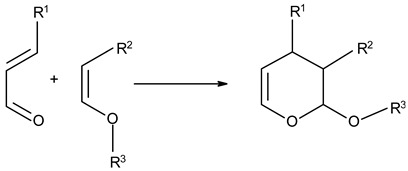
|
No catalyst needed |
| Diels–Alder Reaction |
Diene–Alkene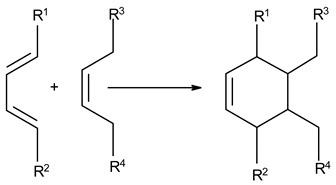
|
No catalyst needed Thermally reversible |
| Thiol-Ene Addition | Alkene–Thiol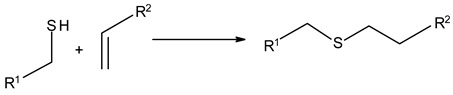
|
Photoinitiator needed Spatial and temporal control |
| Thiol-Michael Addition Click Reactions | Thiol–α,β-Unsaturated carbonyl compound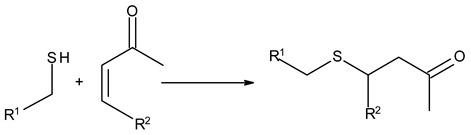
|
No catalyst needed Mild conditions |
| Thiol-Yne Addition | Alkyne–Thiol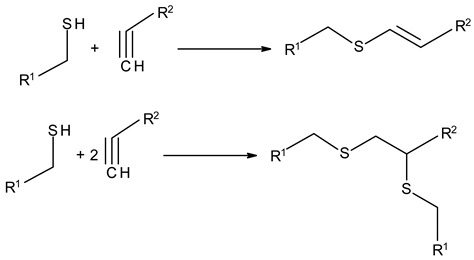
|
Initiator needed |
| Oxime Coupling | Aminooxy compound–Aldehyde/Ketone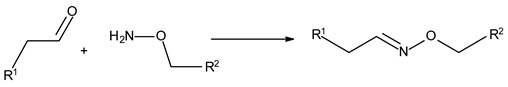
|
No catalyst needed Fast reaction under mild conditions |
