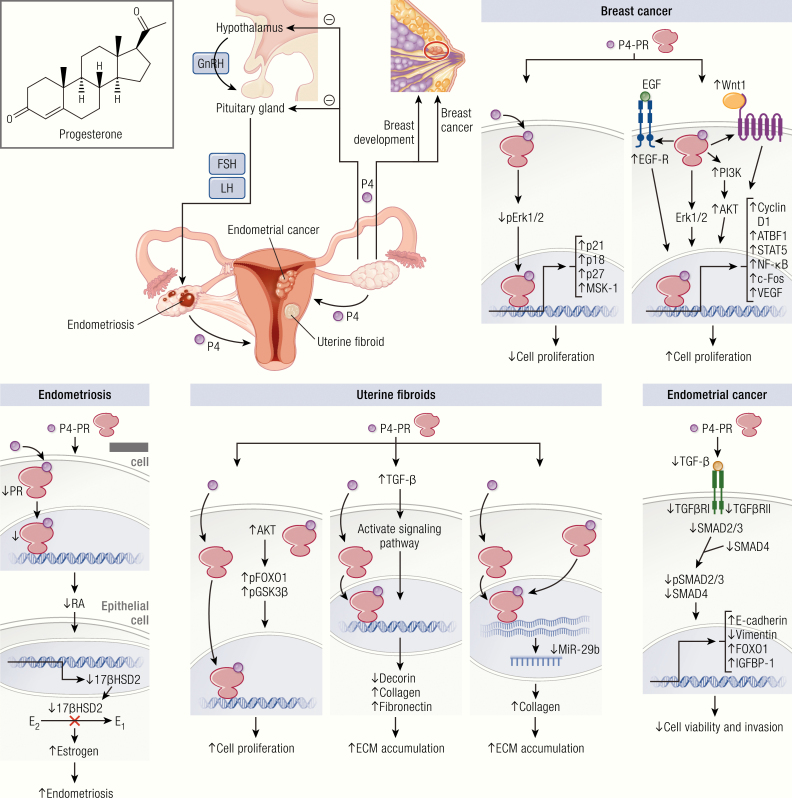
Figure 1.
Progesterone role in reproductive diseases. Progesterone synthesis starts with the signal from the hypothalamus to the pituitary gland to release FSH and LH, which further stimulate ovaries to produce progesterone. Progesterone primarily regulates female reproductive function and breast development. Binding of progesterone at its receptor differentially affects tissue growth at its various sites of action. Progesterone is thought to be stimulatory for uterine fibroid growth, while it is protective for endometrial cancer and endometriosis. The effect of progesterone on breast cancer is complex and variable. Progesterone plays a role in the growth and development of uterine fibroids through stimulation of cell proliferation and facilitating extracellular matrix accumulation. This occurs through activation of the AKT and TGF-β3 pathways and the effects of their downstream intermediaries. Progesterone is thought to negatively impact the development of endometriosis; a reduction in P4-regulated genes and PR-B expression in stromal cells has been reported in endometriotic lesions. Progesterone is thought to play a protective role in the development of some endometrial cancers through downregulation of the TGF-β signaling cascades, with the downstream effect inhibiting the growth of endometrial epithelial cells and reducing cancer cell viability and invasion. In the breast, the role of progesterone is complex and controversial. P4 has been shown to drive proliferation, survival, invasion, and angiogenesis of breast cancer cells through the EGF and Wnt-1 pathway, as well as various other intermediaries. In contrast, progestin has also been shown to induce MKP-1 (MAPK phosphatase 1) expression in a PR-dependent fashion as a means of inducing antiproliferative effects.