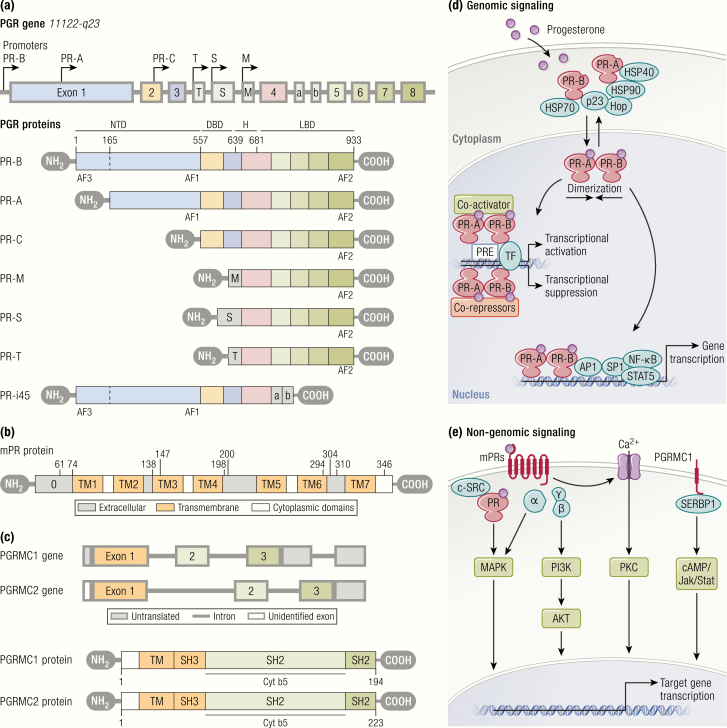
Figure 2.
Progesterone receptors and their activation. A: Structural and functional properties of progesterone receptor isoforms. PR-B is a protein of 933 amino acids, while PR-A lacks 164 amino acids of PR-B at N-terminal region. The common structural elements include highly variable NTD, DBD, H region, and LBD. PR-B consists of 2 transcription activation functions, (AF)-1 and AF-3, but PR-A consists of only AF-1 located at NTD. AF-2 located at LBD presents in both PR isoforms. Hinge region is involved in the binding of DNA and coregulators, and the dimerization of receptors following active transport of PR into the nucleus. Other truncated progesterone receptor isoforms are demonstrated below the shaded box. PR-C contains deletions at the amino terminus that likely result from an alternative location for translation initiation. PR-S and PR-T likely give rise to identical proteins that are truncated at the amino-terminus due to retention of an intronic sequence termed exon S or exon T, respectively. They both retain transcription of H and LBD. PR-M contains a novel 16 amino acid amino-terminal sequence encoded by a sequence in the distal third intron of the PR gene, followed by exons 4 through 8 of the original PR gene. PR-i45 retains 2 intronic sequences termed exons i45a and i45b. This leads to a change in the reading frame, which causes a truncated protein that lacks a functional LBD and DBD. B: Schematic diagram of mPR protein showing extracellular (gray), 7-transmembrane (orange), and cytoplasmic (clear) domains predicted by several programs. C: PGRMC1 is comprised of a single N-terminal TM and a Cyt b5 domain. The protein has sites for interaction with SH (Src-homology)-2 and SH-3 domains of Src tyrosine kinases, kinase binding sites, and a phosphorylation site for tyrosine and serine/threonine kinases. D and E: Progesterone receptor-mediated genomic and nongenomic signaling pathways. Genomic signaling begins with progesterone binding to nuclear receptors (PR-A and PR-B), which induces receptor activation and leads to dissociation with heat shock proteins (HSP90, HSP70, and HSP40), following dimerization and translocation into the nucleus where they bind with PREs within the promoter of target genes. It is the subsequent interaction of the transcription complex with specific coregulators and transcription factors that initiate the transcriptional activation or suppression of target genes. Liganded PR can also activate transcription of genes, the promoters of which lack PREs by acting as a bridge between transcription factors and coactivators at promoters containing activator protein 1 (AP-1), specificity protein1 (Sp1), signal transducer and activator of transcription 5 (STAT5), and NF-κB sites. Progesterone elicits nongenomic actions through binding with membrane-bound progesterone receptors (mPRs: mPRα, mPRβ, and mPRγ; and PGRMC1) or cytoplasmic PRs following association with cytoplasmic kinase cascades (such as cSrc) and downstream signaling pathways. These include (MAPK, Ca2+ infiux/PKC activation, and the PI3K/AKT pathway. P4 exerts nongenomic actions through PGRMC1 via association with SERBP1 and downstream signaling through the cAMP and Jak/Stat kinase signaling pathways.