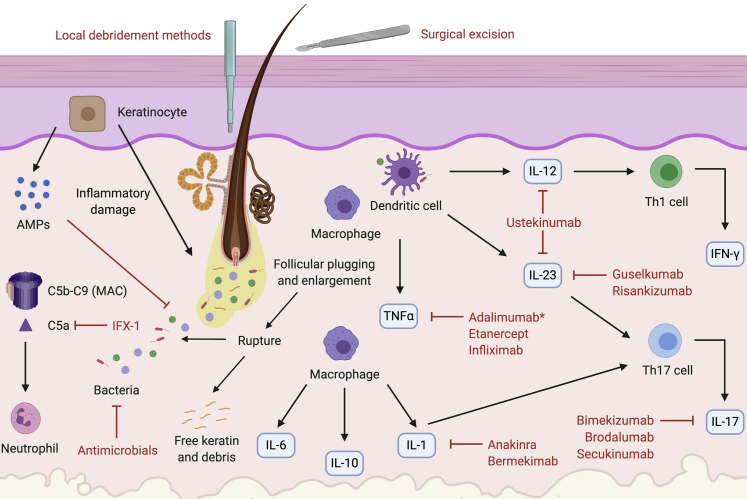
Figure 3.
Targeted therapy for HS according to pathogenesis. Keratinocytes inappropriately release AMPs and proinflammatory cytokines, causing infundibular hyperkeratosis and perifolliculitis (von Laffert et al., 2011). The pilosebaceous unit ruptures, releasing keratin and bacteria, the target of antimicrobial drugs (van der Zee et al., 2012a). Complement is aberrantly activated, increasing levels of C5b–C9, which form the MAC, and C5a, a neutrophil chemotaxin targeted by the mAb IFX-1 (Kanni et al., 2018b). Macrophages are recruited and produce IL-6, IL-10, TNF-α, and IL-1 (van der Zee et al., 2012a). TNF-α is also produced by DCs; it is targeted by the mAbs infliximab and adalimumab and the decoy receptor etanercept (Adams et al., 2010; Grant et al., 2010; Kimball et al., 2016a; Pelekanou et al., 2010). Adalimumab is currently the only FDA-approved mAb therapy for HS (Kimball et al., 2016a). IL-1 is blocked by the mAbs anakinra (targeting IL-1α) and bermekimab (targeting IL-1R) (Kanni et al., 2018a; Tzanetakou et al., 2016). Mature IL-1β, synthesized from the inflammasome, stimulates the development of CD4+ Th17 cells. Activation of these cells is also enhanced by IL-23 (Sabat et al., 2019). CD4+ Th17 cells produce IL-17, which is targeted by the mAbs secukinumab (IL-17A), bimekizumab (IL-17A/IL-17F), and brodalumab (IL-17RA) (Baker and Isaacs, 2018; Thorlacius et al., 2018b). Both macrophages and DCs stimulate production of IL-12 and IL-23. IL-12 drives Th1 differentiation to yield IFN-γ production. IL-23 stimulates Th17 cell differentiation and is targeted by the mAbs guselkumab and risankizumab (Baker and Isaacs, 2018; Kearney et al., 2020; Kovacs and Podda, 2019). Both IL-12 and IL-23 are targeted by the mAb ustekinumab (Blok et al., 2016; Sharon et al., 2012). AMP, antimicrobial peptide; DC, dendritic cell; FDA, Food and Drug Administration; HS, hidradenitis suppurativa; MAC, membrane attack complex; Th, T helper type.