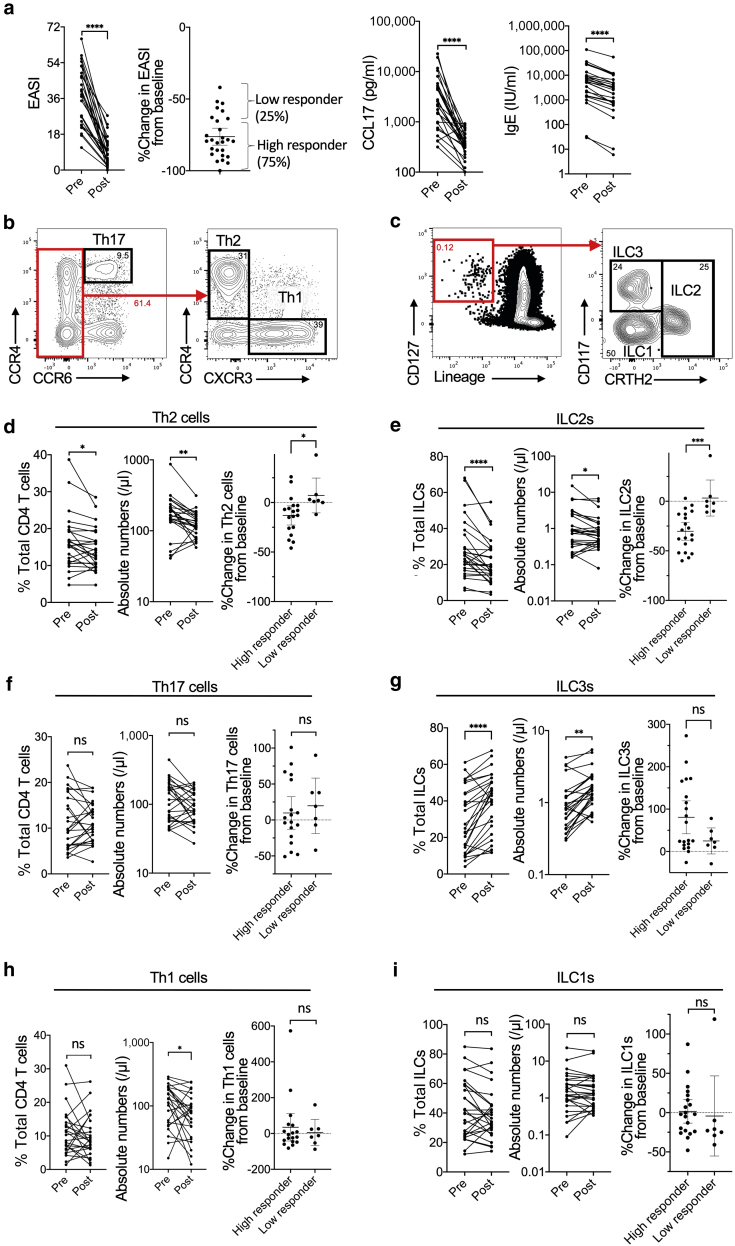
Figure 1.
Changes in clinical data, Th cell populations, and ILC populations before and 16 weeks after initial dupilumab treatment. (a) EASI score of each patient (Pre and Post) and % change from baseline of EASI at week 16. Definition of high responder (75% of total patients) and low responder (25%) of dupilumab is shown. (b) Gating strategy to identify Th2 and Th17 cells. (c) Gating strategy to identify ILC2s and ILC3s. (d) Percentages of Th2 cells among CD4+ T cells, absolute numbers, and % change in Th2 cells from baseline, before (Pre) and 16 weeks after (Post) the dupilumab treatment. (e) Percentages of ILC2s among total ILCs, absolute numbers of ILC2s, and % change in ILC2s from baseline. (f) Percentages of Th17 cells among CD4+ T cells, absolute numbers of Th17 cells, and % change in Th17 cells from baseline. (g) Percentages of ILC3s among total ILCs, absolute numbers of ILC3s, and % change in ILC3s from baseline. Wilcoxon matched-pairs signed rank test or Mann-Whitney test was used to assess statistical significance. ∗∗∗∗P < 0.0001; ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05. Each dot represents a value for each patient. Bold lines represent estimated mean and thin lines indicate the 95% confidence interval. EASI, Eczema Area and Severity Index; ILC, innate lymphoid cell; ns, not significant; Th, T helper type.