Abstract
Bimiralisib is an orally bioavailable pan-phosphatidylinositol 3-kinase and mammalian target of rapamycin inhibitor which has shown activity against lymphoma in preclinical models. This phase I/II study evaluated the response rate to bimiralisib at 2 continuous dose levels (60 mg and 80 mg) in patients with relapsed/refractory lymphoma. Fifty patients were enrolled and started treatment. The most common histologies were diffuse large B-cell lymphoma (n = 17), follicular lymphoma (n = 9), T-cell lymphoma (n = 8), and others (mostly indolent). Patients had been treated with a median of 5 prior lines of treatment and 44% were considered refractory to their last treatment. Mean duration of treatment (and standard deviations) with 60 mg once daily (o.d.) was 1.3 ± 1.2 months, and with 80 mg o.d. 3.7 ± 3.9 months. On an intention to treat analysis, the overall response rate was 14% with 10% achieving a partial response and 4% a complete response. Thirty-six percent of patients were reported as having stable disease. No dose-limiting toxicities were observed during the phase I portion of the study. Overall, 70% of patients had a grade 3 treatment emergent adverse events (TEAE) and 34% had a grade 4 TEAE; 28% of patients discontinued treatment due to toxicity. The most frequent TEAEs grade ≥3 was hyperglycemia (24%), neutropenia (20%), thrombocytopenia (22%), and diarrhea (12%). Per protocol, hyperglycemia required treatment with oral antihyperglycemic agents in 28% and with insulin in 14%. At 60 mg or 80 mg continuous dosing, bimiralisib showed modest efficacy with significant toxicity in heavily pretreated patients with various histological subtypes of lymphoma.
Introduction
Non-Hodgkin lymphoma (NHL) is the commonest hematological malignancy worldwide1 and is composed of over 40 subtypes which can be clinically aggressive or indolent.2 Molecularly targeted therapy of the downstream signaling components of the immunoglobulin receptor has met with considerable success in these disorders. For example, inhibition of Bruton tyrosine kinase and phosphatidylinositol 3-kinase (PI3K) with ibrutinib or idelalisib, respectively, have demonstrated efficacy in chronic lymphocytic leukemia (CLL),3 mantle cell lymphoma (MCL),4 follicular lymphoma,5 and Waldenström macroglobulinemia.6 Mammalian target of rapamycin (mTOR) is a ubiquitous serine threonine kinase involved in regulating protein synthesis and cell growth in response to environmental stimuli.7 It is found within 1 of 2 multiprotein complexes, mTOR complex (mTORC) 1 or 2, and inhibition of mTOR is also of proven, albeit modest, efficacy in relapsed MCL.8 Proven clinical activity, combined with results from a number of preclinical studies demonstrating synergy with PI3K and mTOR inhibitors in hematological malignancies9,10 provided a strong rationale for dual inhibition as a therapeutic strategy in these disorders.
Bimiralisib (PQR309) is an orally bioavailable, selective dual PI3K/mTOR inhibitor11 which has shown activity against a variety of lymphoma cell lines and animal models both as monotherapy and in combination.12 It has pan class 1 PI3K inhibitory activity thereby inhibiting α, β, γ, and δ isoforms in contrast to other PI3K inhibitors which target selective isoforms such as idelalisib (δ-specific13) and duvelisib (γ-δ-specific14). Furthermore, observed mTOR inhibition is balanced with PI3K inhibition and is a direct ATP competitive inhibitory process. Such direct mTOR inhibition is in contrast to the first generation of mTOR inhibitors (such as rapamycin and temsirolimus) which were specific inhibitors of mTORC1 due to their indirect (allosteric) activity via binding of FK506 binding protein 12.15 Dual TORC inhibition could be clinically important as mTORC1 inhibition can lead to direct AKT activation by mTOR within mTORC2,16 thereby generating a possible resistance mechanism.
Bimiralisib has been tested in an accelerated titration, 3 + 3, open label phase I trial of continuous once-daily dosing in solid tumors.17 Twenty-eight patients were enrolled and dosed between 10 mg and 150 mg. The maximum tolerated dose and recommended phase 2 dose from this cohort was 80 mg once daily (o.d.) A single partial response (PR) was observed in a patient with metastatic thymus cancer and 24% disease volume reduction in a patient with sinonasal cancer.
This phase I/II open-label multicentre trial evaluated the efficacy and safety of bimiralisib at different dose levels in patients with relapsed/refractory indolent and aggressive NHL.
Methodology
Eligibility
To be eligible for the trial, patients 18 years or over with an Eastern Cooperative Oncology Group performance status of 0-1 with histologically proven relapsed or refractory lymphoma must have received at least 2 therapeutic lines of prior treatment. Patients with CLL were eligible following at least 1 prior line of standard therapy. Patients with high-grade transformation from an underlying indolent lymphoma or CLL were permitted if they had also failed 1 potentially curative line of therapy. For the phase 2 part of the trial, patients must have clearly measurable disease with a single nodal or extranodal lesion having a long axis diameter of ≥1.5 cm attributable to relapsed lymphoma. Eligible patients were HIV negative, hepatitis C negative, and hepatitis B surface antigen negative. Patients were required to have adequate organ function at screening defined as absolute neutrophil count ≥ 1.0 × 109/L, platelets ≥ 75 × 109/L, hemoglobin ≥ 85 g/L, total bilirubin ≤ 1.5 times the upper limit of normal (ULN) and alanine aminotransferase and aspartate aminotransferase ≤ 2.5 times ULN, serum creatinine ≤ 1.5 times ULN and a fasting glucose < 7.0 mmol/L.
Key exclusion criteria included are listed as follows: (i) immunosuppression due to prior allogenic stem cell transplantation (SCT) or any immunosuppressive therapy within 4 weeks before trial registration, (ii) autologous SCT within 3 months, (iii) any anticancer therapy (radiotherapy, endocrine, investigational, or immunotherapy) within 21 days, (iv) prior grade 4 toxicity from previous exposure to PI3K/mTOR inhibitors, (v) unresolved toxicity from prior therapy grade ≥2 (National Cancer Institute Common Terminology Criteria for Adverse events [NCI-CTCAE] v4.03), (vi) concomitant treatment that increase the pH (reduce acidity) of the upper gastrointestinal tract, including, but not limited to, proton-pump inhibitors, H2-antagonists or antacids, (vii) central nervous system involvement, (viii) clinically significant and uncontrolled major medical condition(s) including but not limited to: serious active infection, bleeding diathesis, symptomatic cardiac failure (New York Heart Association class 3-4), uncontrolled hypertension (blood pressure >150/100 mm Hg), cardiac arrhythmia or psychiatric illness which would limit protocol compliance, (ix) pregnancy, lactating women, or inadequate contraception, (x) major surgery <3 weeks, (xi) uncontrolled diabetes, (xii) refractory nausea and vomiting, chronic gastrointestinal diseases or bowel resection precluding adequate oral medication. All patients provided written informed consent. This study was supported by PIQUR Therapeutics AG. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines (NCT03127020).
The primary endpoint was the best overall response rate (ORR) achieved during bimiralisib monotherapy according to the revised Cheson criteria.18 Treatment was continued until either progression or toxicity. Secondary endpoints included progression-free survival (PFS), time to response (TTR), duration of response (DOR), pharmacokinetics, and standard safety parameters. PFS was defined as time from registration to date of progression or death from any cause. Patients without an event were censored at their latest assessment. DOR was the time from first documented response to time of death or progression. TTR was defined as the time from the date of enrolment to the first documentation of either complete (CR) or PR. Time to event outcomes were assessed using Kaplan Meier survival plots. CR, PR, or stable disease (SD) had to be confirmed with subsequent assessment at least 7 weeks later. Patients were defined as refractory to the most recent prior treatment according to the local investigator decision.
Statistical analysis
Safety run in
Although a formal phase 1 study in solid tumors had been completed,17 a safety run in at 60 mg and 80 mg doses (supplied by PIQUR Therapeutics AG, capsule formulation for oral use, capsule strengths 20 mg and 80 mg) using predefined dose-limiting toxicities (DLT) and a modified 3 + 3 design was followed before the phase II expansion to ensure safety in participants with lymphoma. Standard DLT definitions were used (see Supplemental Digital Content, http://links.lww.com/HS/A204, for further details) and were to be observed within the first 28 days of dosing. The number of DLTs observed at the 80 mg dose determined whether 60 mg or 80 mg was used in the phase II expansion phase (Supplemental Digital Content, Figure 1, http://links.lww.com/HS/A204). In each specified dosing schedule, bimiralisib was dosed orally until disease progression, unacceptable toxicity, death from another cause or patient choice. Patients with SD, PR, or CR remained on study; patients with radiological or clinical progressive disease (PD) were withdrawn.
Expansion phase
An intent-to-treat (ITT) efficacy and safety analysis was performed to include all patients who received ≥1 dose of bimiralisib. Following dose escalation, at least 30 patients with any relapsed or/and refractory lymphoma were to be treated in step 1 with the preferred dosing regimen (daily dosing, schedule A or schedule B, Supplemental Digital Content, Figure 1, http://links.lww.com/HS/A204) and the ORR was to be evaluated. Based on the observed ORR, up to 3 lymphoma subtype-specific indications were planned to be selected for further evaluation in step 2. For each selected subtype lymphoma-specific indication, at least 18 patients (combined from step 1 and step 2) had to be evaluable for efficacy (Supplemental Digital Content, Figure 1, http://links.lww.com/HS/A204). An ORR of at least 30% was considered to be of clinical interest for each individual specific histopathological lymphoma subtype. An exact test for the binomial proportion was used, with a 1-sided significance level of 0.10. To have 80% power to detect a response rate of 30%, 18 patients were needed; the null hypothesis (H0: P = 0.10) would be rejected if the number of responses ≤4. Based on review of all available data, the study steering committee concluded that while there were signs of efficacy, the tolerability of PQR309 treatment should be improved, allowing patients to stay on treatment for longer and thus potentially improving efficacy. Consequently, enrolment into the continuous dosing cohort was stopped. Intermittent dosing schedules were subsequently explored as per study protocol but are not reported in this article. ORR observed in all patients treated on the continuous dosing schedule are reported in this article.
Adverse events were evaluated according to NCI-CTCAE version 4.03. All dose modifications, interruptions, or discontinuations were based on the worst preceding AE grade. Details of dose modification schedule are outlined in the Supplemental Digital Content, Tables S1, S2, http://links.lww.com/HS/A204. Once the bimiralisib dose had been reduced, re-escalation was not permitted. If the administration of bimiralisib was interrupted for reasons other than an AE, then bimiralisib was to be resumed at the same dose. After treatment had commenced, patients requiring a dose delay of >14 consecutive days had to be permanently discontinued from study drug. Specific management of hyperglycemia for patients on study is outlined in the Supplemental Digital Content, Figure S3, http://links.lww.com/HS/A204. Neutropenic fever was managed according to local practice. Primary antiviral, antifungal, and antipneumocystis prophylaxis was not mandated and were given according to local practice. Exploratory outcomes included the effect of bimiralisib on insulin, c-peptide, and glucose levels.
Results
Patient characteristics
Between August 2015 and August 2016, 50 patients were treated. Baseline and disease characteristics are outlined in Table 1. Patients had received a median of 5 (range 1-9) prior regimens with 44% refractory to their most recent line of treatment. Ninety-three percent with B-cell malignancies had received prior rituximab and 96% of all patients enrolled had received prior alkylating agents.
Table 1.
Baseline Patient Characteristics.
| Baseline Patient Characteristics | Treatment Groups | ||
|---|---|---|---|
| Continuous | All Schedules | ||
| 60 mg (n = 8) | 80 mg (n = 42) | Total (n = 50) | |
| Median age, y (range) | 63.5 (34–73) | 58 (32–87) | 58 (32–87) |
| Men, n (%) | 5 (62.5) | 29 (69) | 34 (68) |
| ECOG PS score, n (%) | |||
| 0 | 4 (50%) | 19 (45%) | 23 (46%) |
| 1 | 3 (38%) | 22 (52%) | 25 (50%) |
| 2 | 0 (0%) | 1 (2.4%) | 1 (2%) |
| Missing | 1 (12%) | 0 (0%) | 1 (2%) |
| Caucasian | 8 (100%) | 37 (88%) | 45 (90%) |
| Median time since initial diagnosis, mo (range) | 43.4 (7.6–173.7) | 28.8 (3.1–238) | 30.4 (3.1–238) |
| Median number of prior lines (range) | 5.5 (1–8) | 5 (1–9) | 5 (1–9) |
| Prior rituximab exposure in B-cell malignancies | 7/7 (100%) | 30/33 | 37/40 (93%) |
| Prior alkylator exposure | 7/8 (88%) | 41/42 (98%) | 48/50 (96%) |
| Raised LDH | 6/8 (75%) | 27/42 (64%) | 33/50 (66%) |
| Histology | |||
| Diffuse large B-cell lymphoma | 3 (38%) | 14 (33%) | 17 (34%) |
| Follicular lymphoma | 2 (25%) | 7 (17%) | 9 (18%) |
| Mantle cell lymphoma | 1 (12%) | 3 (7%) | 4 (8%) |
| Marginal zone lymphoma | 0 (0%) | 3 (7%) | 3 (6%) |
| Hodgkin lymphoma | 0 (0%) | 2 (5%) | 2 (4%) |
| PTCL-NOS | 1 (12%) | 3 (7%) | 4 (8%) |
| Other | 1 (12%)a | 10 (24%)b | 11 (22%) |
| Disease status | |||
| Refractory | 3 (37%) | 19 (45%) | 22 (44%) |
| Relapse | 5 (63%) | 23 (55%) | 28 (56%) |
| Raised baseline blood glucose | 0/8 (0%) | 4/41 (10%) | 4/49 (8%) |
| Type 2 diabetes mellitus | 0 (0%) | 1 (2.4%) | 1 (2%) |
aWM/LPL (n = 1).
bRichter transformation (n = 1), AITL (n = 3), CLL (n = 2), LPL/WM (n = 1), PMBCL (n = 2), cutaneous T cell (n = 1).
AITL = angioimmunoblastic T-cell lymphoma; CLL = chronic lymphocytic leukemia; ECOG = Eastern Cooperative Oncology Group; LDH = lactate dehydrogenase; NOS = not otherwise specified; PMBCL = primary mediastinal B-cell lymphoma; PS = performance status; PTCL = peripheral T-cell lymphoma; WM/LPL = Waldenstroms macroglobulinemia/lymphoplasmacytic lymphoma.
Treatment with bimiralisib
The initial safety run in phase is summarized in Supplemental Digital Content, Figure 2, http://links.lww.com/HS/A204. Based on all available data, the investigators and the sponsor concluded that 80 mg o.d. given continuously was tolerated as per protocol, enrolling of patients was started on the dose of 80 mg given continuously once daily.
No safety concerns based on the DLT criteria evaluation with the 80 mg o.d. was observed. However, the Steering Committee requested evaluation of long-term tolerability of PQR309 to adequately evaluate the clinical activity of PQR309 in this patient population for at least 4 months.
A total of 50 patients were treated with either continuous 60 mg o.d. (n = 8) or 80 mg o.d. (n = 42) bimiralisib, respectively. Mean duration of treatment (and standard deviations) with 60 mg o.d. was 1.3 ± 1.2 months and with 80 mg o.d. 3.7 ± 3.9 months.
Safety
All 50 patients enrolled in the study experienced at least 1 treatment emergent AE (TEAE); 70% grade 3 and 27% grade 4 with 14 (28%) patients discontinuing treatment. Table 2 details TEAE seen in 10% or more patients. The most frequent TEAEs were fatigue (50%), hyperglycemia (44%), diarrhea (38%), and nausea (38%). Grade 3 or 4 TEAEs occurring in 2 or more participants were hyperglycemia (22%), neutropenia (20%), thrombocytopenia (14%), diarrhea (12%), rash (8%), anemia (4%), depression (4%), fatigue (4%), and back pain (4%). Other grade 4 TEAEs which occurred in 1 patient each were cardiac arrest, cardiac failure, subdural hematoma, small intestinal perforation, Escherichia coli sepsis, lower respiratory tract infection, lung infection, rhabdomyolysis, and tumor embolism. Fifteen patients (30%) died during the study, 1 of infection, 1 of fungal pneumonia and disease progression, 1 of cardiac failure, 1 of tumor embolism, 1 of small bowel obstruction and disease progression, and 10 of progressive lymphoma. All were judged to be unrelated to study drug.
Table 2.
TEAEs According to Dose and Occurring in ≥10% Patients Across Schedules And Doses.
| TEAEs According to Treatment Schedule | Total (n = 50) | 60 mg (n = 8) | 80 mg (n = 42) |
|---|---|---|---|
| Patients with ≥1 grade 3-4 TEAE | 40 (80%) | 6 (12%) | 34 (68%) |
| Patients with ≥1 TEAE leading to permanent study drug discontinuation (disease progression excluded) | 14 (28%) | 2 (4%) | 12 (24%) |
| Patients with ≥1 TEAE leading to dose reduction | 5 (10%) | 0 | 5 (10%) |
| Patients with ≥1 TEAE leading to dose interruption | 26 (52%) | 2 (4%) | 24 (48%) |
| TEAE Occurring in ≥10% Overall (60 mg and 80 mg) | Grade 1 or 2 | Grade 3 or 4 | Total (n = 50) |
| Hematological | |||
| Neutropenia | 2 (4.8%) | 10 (20.6%) | 12 (24%) |
| Thrombocytopenia | 4 (8%) | 7 (14%) | 11 (22%) |
| Anemia | 8 (16%) | 2 (4%) | 10 (20%) |
| Infection | |||
| Urinary tract infection | 5 (10%) | 1 (2%) | 6 (12%) |
| Gastrointestinal | |||
| Diarrhea | 13 (26%) | 6 (12%) | 19 (38%) |
| Nausea | 19 (38%) | 0 | 19 (38%) |
| Weight decrease | 16 (32%) | 1 (2%) | 17 (34%) |
| Decreased appetite | 14 (28%) | 0 | 15 (30%) |
| Vomiting | 10 (20%) | 0 | 10 (20%) |
| Constipationa | 6 (12%) | 0 | 7 (14%) |
| Abdominal pain | 6 (12%) | 0 | 6 (12%) |
| Dyspepsia | 6 (12%) | 0 | 6 (12%) |
| General/metabolic | |||
| Fatigue | 23 (46%) | 2 (4%) | 25 (50%) |
| Hyperglycemia | 11 (22%) | 11 (22%) | 22 (44%) |
| Rash | 12 (24%) | 4 (8%) | 16 (32%%) |
| Depression | 7 (14%) | 2 (4%) | 9 (18%) |
| Pyrexia | 8 (16%) | 0 | 8 (16%) |
| Pruritus | 7 (14%) | 1 (2%) | 8 (16%) |
| Peripheral edemab | 4 (8%) | 0 | 6 (12%) |
| Dyspneaa | 4 (8%) | 1 (2%) | 6 (12%) |
| Dry skin | 5 (10%) | 1 (2%) | 6 (12%) |
| Back pain | 4 (8%) | 2 (4%) | 6 (12%) |
| Cough | 4 (8%) | 1 (2%) | 5 (10%) |
| Hypoalbuminemia | 4 (8%) | 1 (2%) | 5 (10%) |
a1 patient had missing grade.
b2 patients had missing grade.
TEAE = treatment emergent adverse events.
Hyperglycemia was a relatively frequent event as predicted by the mechanism of action of bimiralisib. This was grade 1 in 4 (8%), grade 2 in 7 (14%), and grade 3 in 11 (22%). Supplemental Digital Content, Figure 3, http://links.lww.com/HS/A204, outlines the protocol defined management of hyperglycemia. In summary, oral antihyperglycemics (SGLT2 inhibitors or metformin) were mandated if the fasting blood glucose was ≥9 mmol/L; interruption of bimiralisib and insulin was required if fasting blood glucose was ≥15 mmol/L for >2 days. Bimiralisib could be restarted at the same dose with continuation of oral antihyperglycemics when blood glucose fell to <9 mmol/L for >2 days. Accordingly, 14 patients (%) commenced oral hypoglycemic agents when on study with 6 going onto receive insulin. Seven (14%) patients in total commenced insulin with 1 patient receiving insulin without prior or concomitant oral antihyperglycemic agent. Only 1 patient had a raised fasting blood sugar at baseline.
TEAEs leading to dose discontinuation (excluding disease progression) were diarrhea, fatigue, rhabdomyolysis (n = 2 each) and subdural hematoma, bile duct stenosis, weight decrease, decreased appetite, memory impairment, and pneumonitis (n = 1 each).
Efficacy
On an ITT basis, the best ORR across all patients was 14%. CR was observed as best response in 2 (4%) patients and PR in 5 patients (10%) (Table 3). The ORR for 9 FL patients (89% prior alkylator, 100% prior rituximab) was 11% (1 CR), 7 FL patients had a best response of SD (including 2 patients had a single, unconfirmed PR). Three patients stopped due to PD, 5 due to a TEAE, and 1 was bridged to an allogenic SCT. Of the 32 patients with “aggressive” histology, ORR was 13% (3 PR, 1 CR) and in 18 patients with “indolent” histology, ORR was 17% (2 PR, 1 CR). There was no clear association noted between dosing schedule and response (Supplemental Digital Content, Table S3, http://links.lww.com/HS/A204).
Table 3.
Overall Response Rate According to Histology.
| Histology | Number | Stable Disease | Partial Response | Complete Response | Overall Response Rate (%) |
|---|---|---|---|---|---|
| All patients | 50 | 18 (36%) | 5 (10.5%) | 2 (4%) | 7 (14%) |
| Aggressive | |||||
| Total | 32 | 8 | 3 | 1 | 4 (13%) |
| DLBCL | 17 | 4 | 1 | 1 | 2 (12%) |
| MCL | 4 | 3 | 0 | 0 | 0 (0%) |
| PTCL | 4 | 0 | 0 | 0 | 0 (0%) |
| AITL | 3 | 1 | 1 | 0 | 1 (33%) |
| Cut TCL | 1 | 0 | 0 | 0 | 0 (0%) |
| PMBCL | 2 | 0 | 0 | 0 | 0 (0%) |
| Richters | 1 | 0 | 1 | 0 | 1 (100%) |
| Indolent | |||||
| Total | 18 | 10 | 2 | 1 | 3 (17%) |
| FL | 9 | 7 | 0 | 1 | 1 (11%) |
| LPL/WM | 2 | 1 | 0 | 0 | 0 (0%) |
| CLL | 2 | 1 | 1 | 0 | 1 (50%) |
| cHL | 2 | 0 | 0 | 0 | 0 (0%) |
| MZL | 3 | 1 | 1 | 0 | 1 (33%) |
AITL = angioimmunoblastic T-cell lymphoma; cHL = classical Hodgkin lymphoma; CLL = chronic lymphocytic leukemia; Cut TCL = cutaneous T-cell lymphoma; DLBCL = diffuse large B-cell lymphoma; FL = follicular lymphoma; LPL/WM = lymphoplasmacytic lymphoma/Waldenstroms macroglobulinemia; MCL = mantle cell lymphoma; MZL = marginal zone lymphoma; PMBCL = primary mediastinal B-cell lymphoma; PTCL = peripheral T-cell lymphoma.
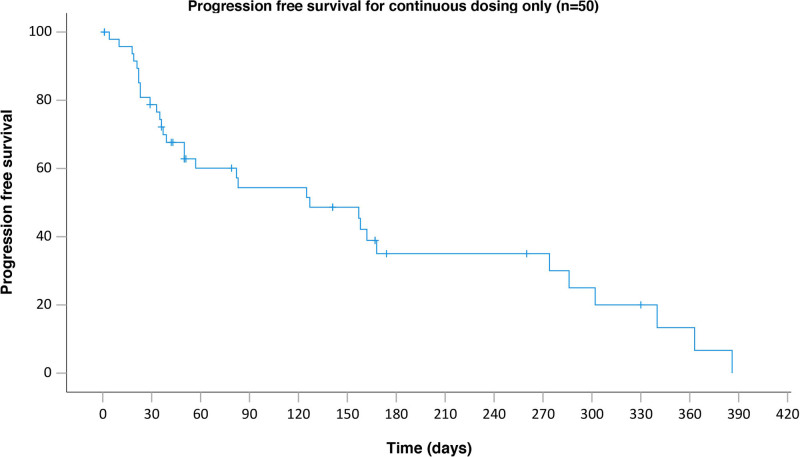
Median TTR across 7 responding patients was 3.5 months (range 1.2-7.2 months). Response data were not available for 9 patients (18%) and 4 patients (8%) were not evaluable and were considered nonresponders. The median PFS was 4.2 months (95% CI, 1.6-6.7) (Figure 1). There was a total of 15 deaths, with the majority (n = 10) related directly to progressive lymphoma. There was no treatment-related mortality (Supplemental Digital Content, Table S4, http://links.lww.com/HS/A204).
Figure 1.
PFS of the whole cohort. PFS = progression-free survival.
Discussion
In this phase I/II study of bimiralisib in heavily pretreated (5 median lines of prior therapy) patients with a variety of histological subtype of relapsed/refractory lymphoma, only modest efficacy was demonstrated with a best response of SD in 36%, PR in 10%, and complete response in 4%. This compares unfavorably with the licensed PI3K inhibitors idelalisib and copanlisib which are associated with ORRs of 43.7% in a mixed relapsed indolent aggressive cohort19 and 57% in relapsed indolent NHL, respectively.5 While the cohort in this study can be regarded as high risk with 34% having relapsed DLBCL, 16% relapsed peripheral T-cell lymphoma and 8% relapsed mantle lymphoma, median prior lines of therapy of 5 and 44% being judged as having refractory disease, within the relapsed follicular lymphoma cohort of 9 patients, 1 CR and 2 unconfirmed PRs were observed. While this demonstrates a signal of efficacy, the benefits seen fail to outweigh the toxicity issues seen with this agent with continuous dosing strategies. It remains an unanswered question whether similar efficacy compared with licensed PI3K inhibitors will be observed in histologies such as follicular lymphoma if the tolerability profile of this agent can improved.
Toxicity was notable for a high frequency (48% all grade) and relative severity of hyperglycemia with 14 patients requiring oral antihyperglycemic agents and 7 patients requiring insulin. Hyperglycemia was an expected on-target toxicity with this agent due to inhibition of both PI3K and mTOR. Previous studies have shown that inhibition of the α isoform of PI3K is particularly linked with hyperglycemia as it mediates virtually all cellular responses to insulin.20 Inhibition of insulin responsiveness would therefore be expected to lead to glycogen breakdown by the liver and impaired glucose uptake by cells, both contributing to raised glucose levels in the blood. Specific PI3K α isoform inhibitors, such as alpelisib, have been tested in the clinic for patients with solid tumors and hyperglycemia was a frequent AE, observed at all grades in 50% of patients.21 Copanlisib is a pan PI3K inhibitor with particular activity against the α and δ isoforms given on days 1, 8, and 15 of a 28-day cycle intravenously and which has received US Food and Drug Adminstration approval for patients with relapsed follicular lymphoma who had received at least 2 prior therapies. This agent has reported a hyperglycemia incidence of 50%, albeit with the phenomenon resolving generally within 24 hours of dosing.22 mTOR inhibition is also associated with hyperglycemia albeit less marked than with PI3K α inhibitors. In 2 large placebo controlled studies in metastatic renal cell carcinoma for example, single agent everolimus was associated with all grade hyperglycemia of 20% and 6%, respectively.23
One strategy to potentially reduce toxicity is to use an intermittent rather than continuous dose schedule. Indeed, one possible reason for the transient nature of the copanlisib-induced hyperglycemia maybe the intermittent dose schedule of day 1, 8, and 15 of a 28-day cycle. Preliminary data from the PI3 kinase delta selective inhibitor ME-401 (zandelisib) have been presented using an intermittent schedule of 7 days on drug and 21 days off drug following 2 cycles of continuous dosing for a total of 56 days. A low rate of delayed grade 3 AEs were seen once patients had switched to the intermittent schedule.24 Intermittent dose schedules of duvelisib, the gamma delta PI3 kinase inhibitor are also being investigated.25 In line with the above, and based on initial safety signals an intermittent dose study of bimirasilib administered day 1, day 2 weekly is being planned.
In conclusion, at continuous doses of 80 mg and 60 mg, the orally bioavailable pan PI3K and mTOR inhibitor bimiralisib showed only modest response but significant toxicity in 50 patients with heavily pretreated relapsed or refractory lymphoma of various histological subtype.
Acknowledgments
The team thank all the patients involved in the study and their families. We thank Jeremy Levy for his statistical input. TAE acknowledges support from the Julian Starmer-Smith lymphoma fund. GPC and TAE acknowledge support from the Haematology and Stem cells theme of the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Programme and the CRUK Experimental Cancer Medicines Centre (ECMC). RP is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. WT is supported by funding from the NIHR University College London Hospitals Biomedical Research Facility. The views expressed are those of the authors and not necessarily those of the funding bodies. PQR309 (Bimiralisib) was provided free of charge by PIQUR. DC receives funding from the NIHR BRC at the Royal Marsden and Institute of Cancer Research. The authors would like to thank Dr Matthew Wilson, Queen Elizabeth University Hospital, Glasgow for his statistical assistance.
Sources of funding
This trial is funded by PIQUR who provided free of charge Bimiralisib.
Disclosures
GPC has Scientific advisory board honorarium from Roche, Gilead, Pfizer, Takeda, Beigene, Incyte, Daiichi Sankyo, Celleron, and ADCT Therapeutics, Speaker fees from Roche, Takeda, Gilead, Novartis, BMS and research funding from: BMS, MSD, Pfizer, Amgen, Celleron. TAE has received from Roche: Honorarium, Advisory Board Honorarium, from Gilead: Honorarium; Research support; Travel to scientific conferences, from KITE: Advisory Board Honorarium, from Takeda: Travel to scientific conferences, from Janssen: Honorarium, from Abbvie: Honorarium; Travel to scientific conferences, from AstraZeneca: Honorarium, Research funding, fromLoxo Oncology: Advisory Board Honorarium, Trial steering committee, from Beigene: Advisory Board Honorarium and research funding, from Incyte: Advisory Board Honorarium. MD has received research funding from Abbvie, Bayer, Celgene, Janssen, and Roche, Speakers Honoraria from Bayer, Celgene, Gilead, Janssen, Roche, Scientific Advisory Board Honorarium from Astra Zeneca, Bayer, Beigene, Celgene, Gilead, Janssen, Novartis, and Roche. WT has received honoraria and consultancy fees from Roche, Gilead Sciences, and Celgene. AS has provided consultancy services to Bayer, Eli Lilly; has received institutional research funding from PIQUR, Bayer, Roche, Pfizer, Merck, Novartis, MEI Therapeutics, ADC Therapeutics and Abbvie; has received travel grants from Abbvie and PharmaMar. DC has received Research funding from Amgen, Sanofi, Merrimack, AstraZeneca, Celgene, MedImmune, Bayer, 4SC, Clovis, Eli Lilly, Janssen and Merck and has received Scientific Advisory Board honorarium from OVIBIO. LG-R: Janssen and ADC Therapeutics: consulting. DS-R, SD and MR are state (former) employees of PIQUR Therapeutics. All the other authors have no conflicts of interest to disclose.
Supplementary Material
Footnotes
Ethical approval: EudraCT: 2015-001306-33 (PQR309-002) and 2016-000125-38 (PQR309-002A).
Clinical trial ID: NCT03127020.
Supplemental digital content is available for this article.
References
- 1.Miranda-Filho A, Piñeros M, Znaor A, et al. Global patterns and trends in the incidence of non-Hodgkin lymphoma. Cancer Causes Control. 2019;30:489–499. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Tedeschi A, Trotman J, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenström’s macroglobulinemia. N Eng J Med. 2018;378:2399–2410. [DOI] [PubMed] [Google Scholar]
- 7.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. [DOI] [PubMed] [Google Scholar]
- 8.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Xiao X, Meng X, et al. A mechanism for synergy with combined mTOR and PI3 kinase inhibitors. PLoS One. 2011;6:e26343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JS, Tang SS, Ortiz V, et al. MCL-1-independent mechanisms of synergy between dual PI3K/mTOR and BCL-2 inh in diffuse large B cell lymphoma. Oncotarget. 2015;6:35202–35217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaufils F, Cmiljanovic N, Cmiljanovic V, et al. 5-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine (PQR309), a potent, brain-penetrant, orally bioavailable, pan-class I PI3K/mTOR inhibitor as clinical candidate in oncology. J Med Chem. 2017;60:7524–7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarantelli C, Gaudio E, Arribas AJ, et al. PQR309 is a novel dual PI3K/mTOR inhibitor with preclinical antitumor activity in lymphomas as a single agent and in combination therapy. Clin Cancer Res. 2018;24:120–129. [DOI] [PubMed] [Google Scholar]
- 13.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkler DG, Faia KL, DiNitto JP, et al. PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013;20:1364–1374. [DOI] [PubMed] [Google Scholar]
- 15.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. [DOI] [PubMed] [Google Scholar]
- 16.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wicki A, Brown N, Xyrafas A, et al. First-in human, phase 1, dose-escalation pharmacokinetic and pharmacodynamic study of the oral dual PI3K and mTORC1/2 inhibitor PQR309 in patients with advanced solid tumors (SAKK 67/13). Eur J Cancer. 2018;96:6–16. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28:2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins BD, Pauli C, Du X, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juric D, Rodon J, Tabernero J, et al. Phosphatidylinositol 3-kinase α-selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: results from the first-in-human study. J Clin Oncol. 2018;36:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreyling M, Santoro A, Mollica L, et al. Long-term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2-year follow-up of the CHRONOS-1 study. Am J Hematol. 2020;95:362–371. [DOI] [PubMed] [Google Scholar]
- 23.Bono P, Oudard S, Bodrogi I, et al. Outcomes in patients with metastatic renal cell carcinoma who develop everolimus-related hyperglycemia and hypercholesterolemia: combined subgroup analyses of the RECORD-1 and REACT trials. Clin Genitourin Cancer. 2016;14:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelenetz AD, Soumerai JD, Jagadeesh D, et al. Preliminary safety and efficacy results with an intermittent schedule of the PI3kδ inhibitor ME-401 alone or in combination with rituximab for B-cell malignancies. Blood. 2018;132(suppl 1):2893–2893. [Google Scholar]
- 25.Karmali R, Youssoufian H, Sprott K, et al. A phase 2, randomized, open-label, 2-arm study comparing 2 intermittent dosing schedules of duvelisib in patients with indolent non-Hodgkin lymphoma (iNHL) (TEMPO). Blood. 2019;134(suppl_1):5251–5251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.