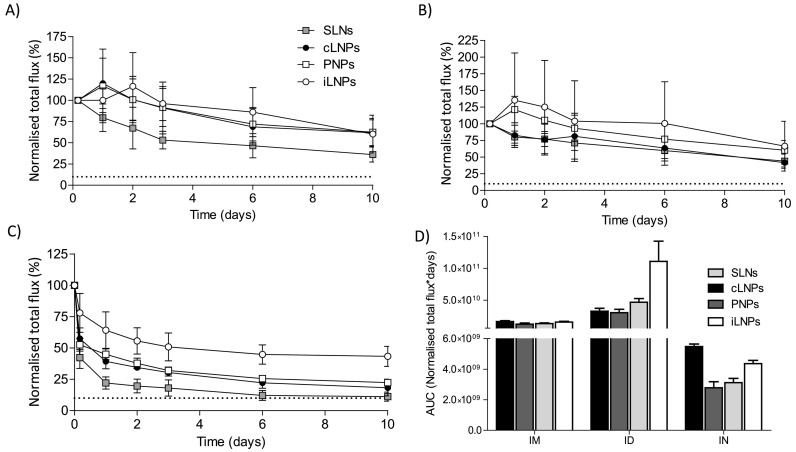
Fig. 6.
Pharmacokinetic profile at the site of injection of RVG-saRNA loaded SLNs, PNPs and LNPs. Pharmacokinetic profile at the site of injection of either saRNA-SLNs, saRNA-PNPs or saRNA-LNPs following A) intramuscular, B) intradermal or C) intranasal administration. Mice received 25 μg of nanoparticles, corresponding to the administration of 1 μg of saRNA. A naive mouse was used as a negative control. D) Calculated areas under the curve at the site of injection for saRNA encapsulating LNPs, PNPs and SLNs administered by intramuscular (IM), intradermal (ID) or intranasal (IN) route. The total flux was normalised by dividing each time point by the value at 4 h time point as it was the highest in each group. This was considered as 100%Dotted line represents the background value. Results are represented as mean ± SD of five animals per group.