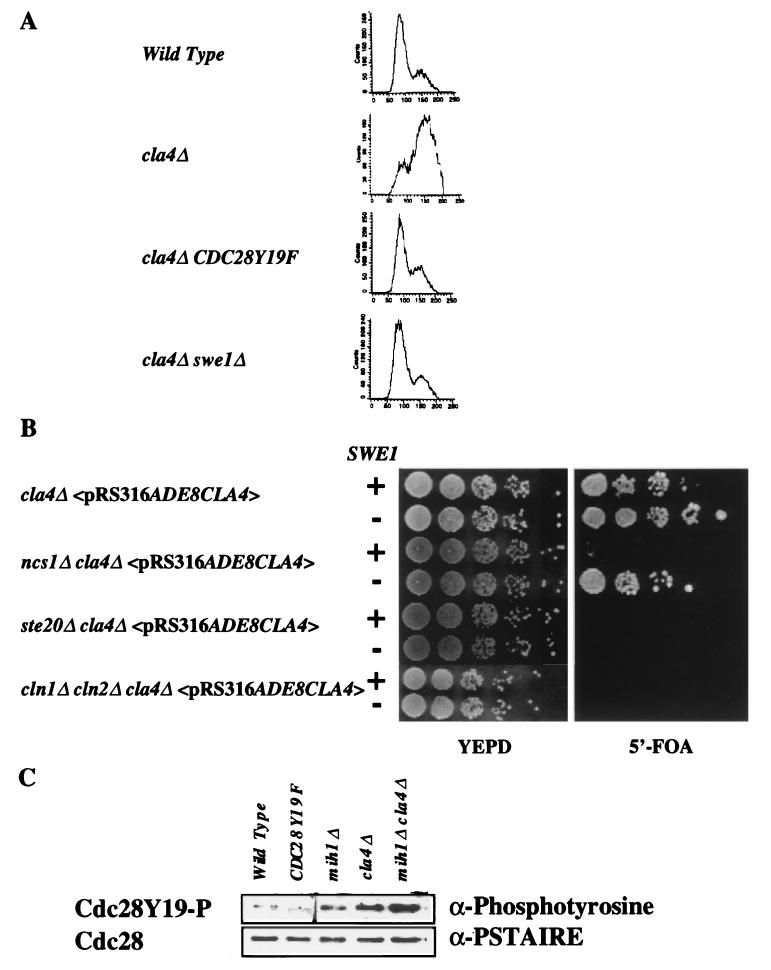
FIG. 4.
The cla4Δ ncs1Δ::LEU2 defect results in a G2 delay that is suppressed by deletion of SWE1. (A) Strains SY3357, SY3360, SY3392, and SY3391 were grown to mid-log phase in YEPD medium at 30°C. The cells were fixed, stained with propidium iodide, and subjected to FACS analysis. (B) Strains expressing wild-type SWE1 (SY3362, SY3378, SY3368, and SY3504) or lacking SWE1 (SY3392 carrying pRS316ADE8CLA4, SY3403, SY3404, and SY3505) were constructed and grown on YEPD medium at 25°C. After 3 days, dilutions of cells were spotted onto rich medium (YEPD) or onto synthetic complete medium supplemented with 5′-FOA and incubated for 3 days at 25°C. (C) Deletion of CLA4 increases the phosphotyrosine content of Cdc28p. Whole-cell extracts were prepared from exponentially growing strains SY3357, SY3390, SY3393, SY3360, and SY3398. Cdc28p was enriched from the extracts using p13SUC1-conjugated agarose beads. The beads were washed, and the eluted proteins were analyzed by Western analysis using antibodies that recognize phosphotyrosine (4G18) and the PSTAIRE amino acid motif of Cdc28p. The band that appears in the Cdc28Y19F sample of the upper panel (antiphosphotyrosine blot) differs in mobility from Cdc28p. We therefore conclude that it is not Cdc28p but another protein that is phosphorylated on a tyrosine residue(s), but only in Cdc28Y19F cells.