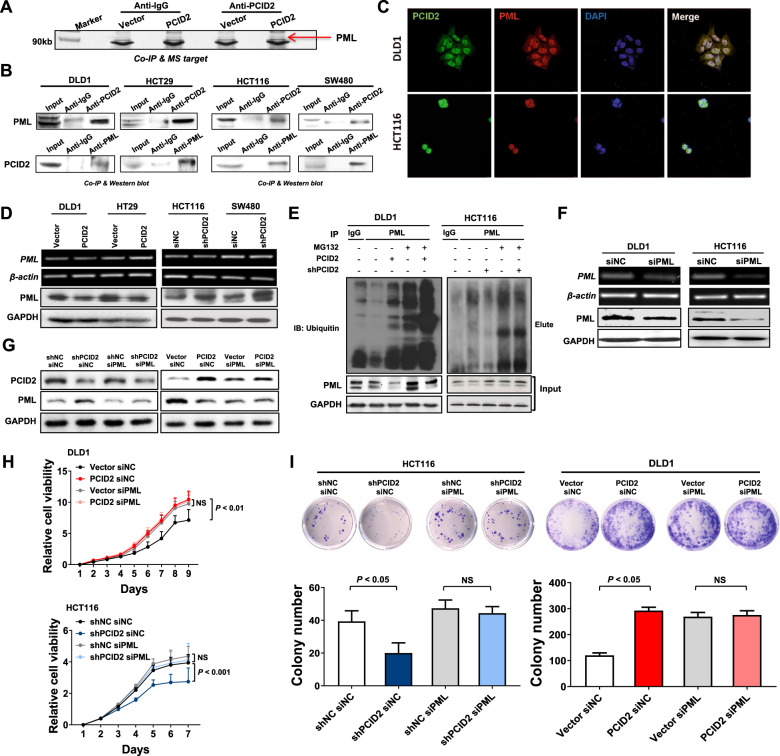
Fig. 5. PCID2 interacts with promyelocytic leukemia (PML) by mediating the degradation of PML.
A Immunoprecipitant of PCID2 was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and proteins were stained by silver staining. Proteins of interest were identified by mass spectrometry (indicated by arrow). B The interaction between PCID2 and PML was confirmed by co-immunoprecipitation (IP). C PCID2 and PML are co-localized in the nucleus as demonstrated by confocal immunofluorescence microscopy. D Ectopic expression or knockdown of PCID2 did not alter mRNA expression of PML, but decreased and enhanced protein expression of PML, respectively. E Ectopic expression of PCID2 promoted polyubiquitination of PML. Cell lysates were subjected to immunoprecipitation (IP) with anti-PML antibody or rabbit IgG as control, followed by immunoblotting with respective antibodies as indicated. Smear bands corresponds to polyubiquitinated PML. F Knockdown of PML in DLD1 and HCT116 cells was confirmed by RT-PCR and Western blot. G Protein expression of PCID2 and PML were checked by Western blot. H Knockdown of PML abolished the growth-promoting effect mediated by PCID2 determined by cell viability assay. I Knockdown of PML significantly abolished the promoting effect of PCID2 on clonogenicity. Two-tailed unpaired t test, NS not significant.