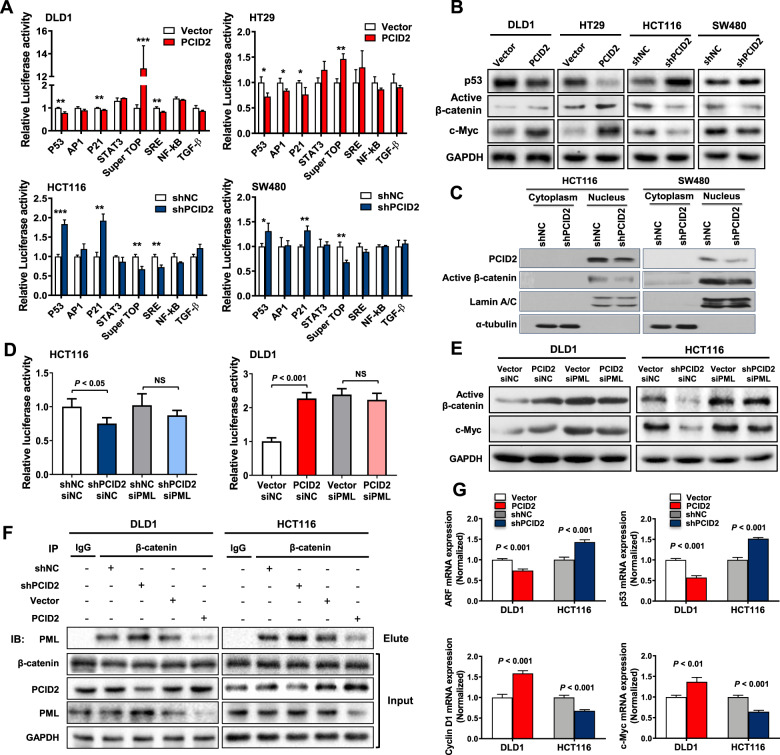
Fig. 6. PCID2 mediated the degradation of PML, resulting in the bi-directional regulation of canonical and non-canonical Wnt signaling pathways.
A Luciferase reporter assay for eight major cancer-related pathways was performed in PCID2-overexpressed and PCID2-silenced CRC cells. B Protein expression of active β-catenin, c-Myc and p53 was determined by Western blot. C PCID2 is localized in the nucleus and PCID2 knockdown reduced the expression of active β-catenin as demonstrated by Western blot of cytoplasmic and nuclear fractions. D, E Knockdown of PML abrogated the effect of PCID2 on activation of Wnt signaling pathway. F The interaction between PML and β-catenin was determined by IP assay. Depletion and overexpression of PCID2 increased and decreased the binding of PML to β-catenin, respectively. G mRNA expression of downstream effectors of non-canonical β-catenin signaling (ARF and p53) and canonical Wnt/β-catenin signaling (Cyclin D1 and c-Myc) was determined by qPCR. Two-tailed paired t test, NS not significant, *P < 0.05, **P < 0.01, ***P < 0.001.