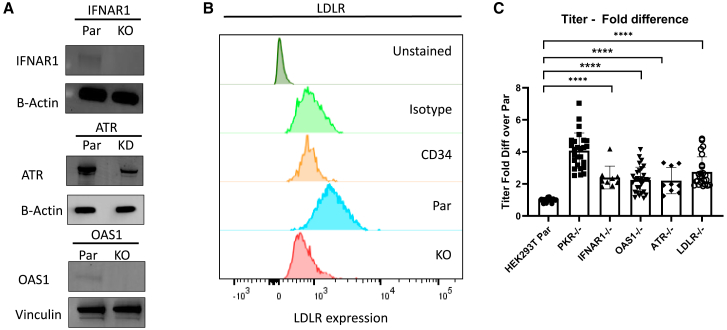
Figure 1.
Knocking out IFNAR1, ATR, OAS1, and LDLR in 293T cells increased titers
(A) IFNAR1, ATR, and OAS1 protein expression in parental and gene-edited cells measured by western blot. HEK293T cells were edited by CRISPR-Cas9 targeting each of the genes. The edited cells were sorted for single-cell clones, and an isogenic clone with no parental allele, except ATR, was expanded for protein expression analysis. Because ATR is an essential gene for cell survival, an ATR KD clone with 33% parental allele remaining was selected. β-Actin and Vinculin were used as the loading controls. (B) LDLR protein expression in parental, LDLR−/− 293T cells, and unstimulated CD34+ HSPCs measured by flow cytometry. Unstimulated CD34+ HSPCs are known to not express LDLR and were used as a negative control. A mouse IgG1 antibody was used as the isotype control. (C) Titers of Lenti/βAS3-FB packaged in parental and gene-edited cells (n = 9–29 dishes of identical cultures from three to 10 independent experiments; bars represent mean with SD; unpaired t test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). PKR−/− cells are known to increase LV titers and were used as a positive control to evaluate titer increase. LVs in unconcentrated viral supernatant were assayed for titer by transducing HT29 cells at 10-fold serial dilution and VCN measured by ddPCR.