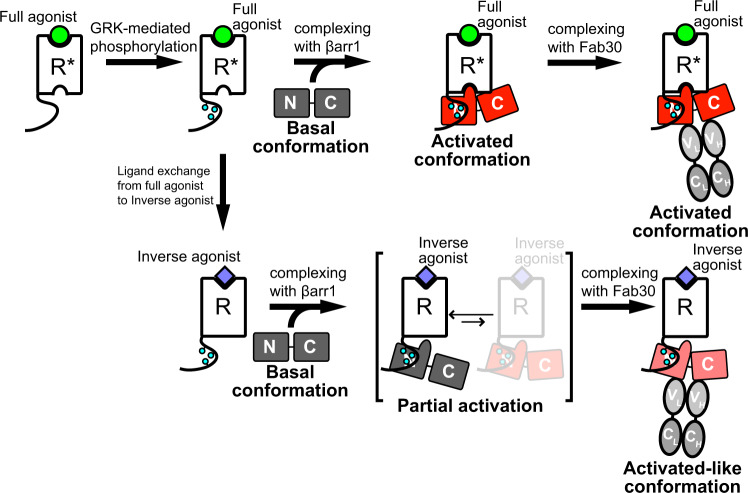
Fig. 5. Conformational activation of βarr1 upon binding to GPCRs and the synthetic antibody fragment Fab30.
The full agonist-bound receptor is phosphorylated by GRK. The phosphorylated and full agonist-bound receptor interacts with βarr1 through both the TM core and C tail, and βarr1 in the complex adopts the activated conformation. Fab30 binding to the complex does not induce significant conformational changes in βarr1. In the case where the full agonist is replaced with the inverse agonist after GRK-mediated phosphorylation, the receptor only interacts with βarr1 through the C tail, resulting in partial activation in which βarr1 exists in equilibrium between the basal and activated conformations. Fab30 binding to the complex promotes βarr1 to adopt the activated-like conformation, which is partly similar to the activated conformation.