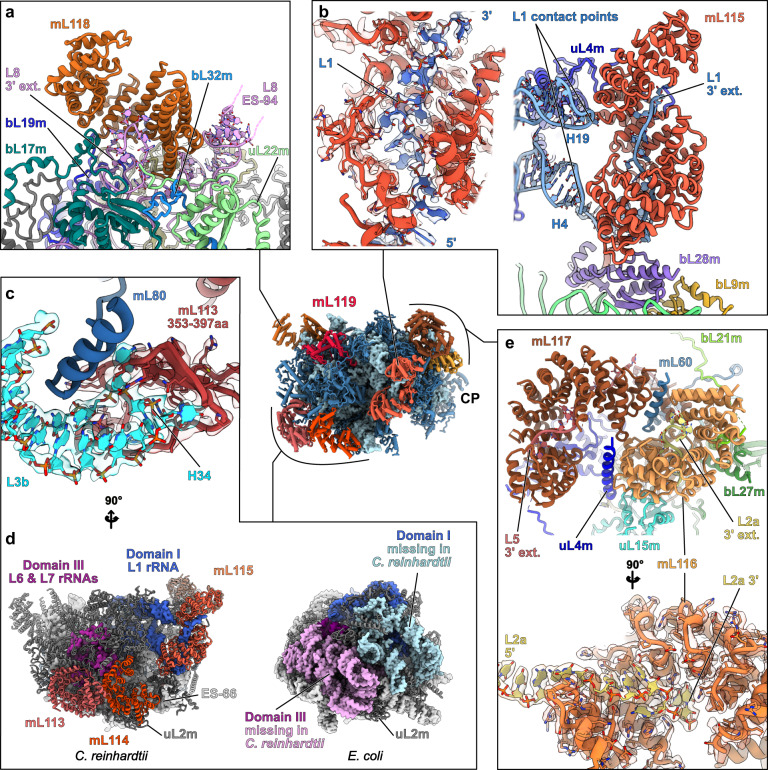
Fig. 4. Chlamydomonas-specific proteins stabilize the fragmented rRNAs via highly intertwined interactions.
Magnified views of the Chlamydomonas-specific r-proteins involved in rRNA stabilization. a mL118 stabilizes the 3′ end of the L8 fragment and contacts the expansion segment 94 (L8 ES-94). b mL115 stabilizes the highly reduced domain I, which is formed by the L1 fragment. mL115 binds the 3′ end of the L1 fragment and also contacts the L1 fragment at two specific points corresponding to H4 and H19. The single-stranded portion of L1 interacting with mL115 is shown in its density. c Detailed view of the mL113 contact with the L3b fragment. An inter-repeat domain of mL113 (red) formed by amino acids 353–397 clamps the tip of H34 (cyan). The models are shown in their densities. Contrary to the rest of the r-proteins, mL113 does not enlace single-stranded rRNA. d Structural compensation for the loss of large portions of domain I and III. The missing rRNA regions are depicted on the E. coli model in pink and light blue, and the compensating proteins mL113, mL114, and mL115 (red shades) are shown on the Chlamydomonas model. mL113 and mL114 compensate for domain III reduction, and mL115 stabilizes and compensates for the reduced domain I. e mL116 and mL117 interact with each other and with several surrounding proteins. mL117 is involved in stabilizing the 3′ end of the L5 rRNA fragment, and mL116 stabilizes the L2a 3′ extremity, but also makes additional contacts with rRNAs (Supplementary Fig. 4). The single-stranded portion of L2a in mL116 is shown in its density. With the exception of the mTERF protein mL114, the rest of the proteins belong to the same class of ASA2-like/OPR proteins. Further detailed views are shown in Supplementary Fig. 4.