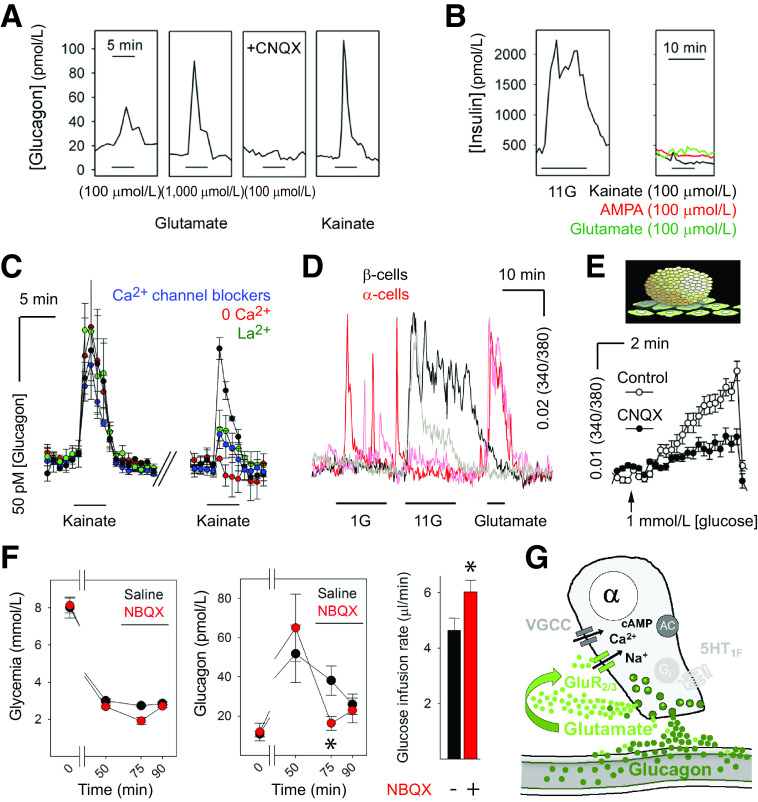
Figure 3.
Glutamate receptor signaling forms a positive autocrine loop that amplifies glucagon secretion. A: Glutamate induced glucagon responses in human islets that could be blocked by the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μmol/L). The iGluR agonists kainate and AMPA (both 100 μmol/L) also elicited strong glucagon secretion. B: Insulin release was induced by high glucose (11 mmol/L; 11G) but not by kainate (representative of six islet preparations). C: Glucagon secretion from human islets in response to kainate was blocked by Ca2+ channel blockers and was strongly reduced in the absence of nominal Ca2+ in the solution. D: Ca2+ imaging of dispersed human β-cells (black traces) and α-cells (red traces) showed that only α-cells responded to glutamate. E: Glucagon secretion in response to a drop from 11 to 1 mmol/L glucose concentration, as measured by biosensor cells, was inhibited in the presence of CNQX (10 μmol/L). F, left: Hyperinsulinemic-hypoglycemic clamp to provide a constant hypoglycemic stimulus at ∼3 mmol/L blood glucose concentration was induced with insulin infusion in mice. Glucagon secretion in response to hypoglycemia was significantly diminished in mice after infusion of the iGluR antagonist NBQX (10 mg/kg; red symbols; n = 7) compared with saline-infused mice (black symbols; n = 3; repeated-measures ANOVA, P < 0.05). Bar indicates drug infusion. Notice that the glucose infusion rate needed to maintain glycemia after drug infusion was significantly larger in NBQX-treated mice than in saline-treated mice (not shown; n = 3; Student’s t test, P < 0.05). G: Model of how α-cells release glutamate to amplify glucagon secretion in human islets. GluR2/3, iGluRs of the AMPA type composed by GluR2 and GluR3 subunits; VGCC, voltage-gated Ca2+ channels. Data werae adapted from Cabrera et al. (31).