Abstract
ImmunoCard STAT! E. coli O157:H7 (Meridian Diagnostics, Inc., Cincinnati, Ohio) is a novel rapid (10-min) test for the presence of Escherichia coli O157:H7 in stools. The test may be performed either directly on stool specimens or on an overnight broth culture of stool. In a multicenter prospective study, 14 of 14 specimens positive by culture for E. coli O157:H7 were positive by the ImmunoCard STAT! O157:H7 test, and there were no false positives from 263 culture-negative specimens. In a retrospective study, the test was positive in 339 (81%) of 417 stored culture-positive specimens and the specificity was 95% (98 of 103 specimens). No false positives were associated with alternate stool pathogens. The ImmunoCard STAT! O157:H7 test has high sensitivity and specificity.
Enterohemorrhagic Escherichia coli (verotoxigenic E. coli [VTEC]) is an important cause of diarrhea. This organism produces toxin (verotoxin or Shiga-like toxin). Most patients with VTEC infection recover uneventfully within a few days, but in some cases (≅8%) (10) the diarrhea is followed by hemolytic-uremic syndrome, a life-threatening complication with substantial morbidity in survivors (6, 11). Worldwide, many serotypes of VTEC have been described, but in North America one type predominates, E. coli O157:H7 (2, 3). Because of the potential seriousness of VTEC infection (1), there is a need for rapid tests for the presence of VTEC which can be directly applied to stool specimens. Prospective and retrospective studies using ImmunoCard STAT! E. coli O157:H7 (ICS; Meridian Diagnostics, Inc., Cincinnati, Ohio), a new test for O157:H7 VTEC which detects both O157 lipopolysaccharide and H7 protein antigen, are described here.
MATERIALS AND METHODS
ICS test.
The test kit consists of a plastic holder with a well in which the stool sample or broth culture is placed (Fig. 1). The test incorporates two antibodies, one against lipopolysaccharide O157 and the other against flagellar antigen H7. A suspension of stool (or broth culture) is placed on the sample pad, under which there is a conjugate pad impregnated with anti-O157 antibody bound to colloidal gold. Approximately halfway along, the membrane is impregnated with a transverse band of anti-H7 antibody, and at the distal end of the membrane is a second band consisting of anti-globulin. When E. coli O157:H7 is present in the specimen the organisms bind to the colloidal gold particles which then diffuse to the backing material and along the nitrocellulose membrane towards the distal end. If the stool is O157:H7 positive, the colloidal gold particles, with the H7 antigen attached, are bound by the H7 antibody strip and show as a purple band. The distal band recognizes the colloidal gold–anti-O157 conjugate and serves as a control to indicate that the colloidal gold conjugate is active and that diffusion through the paper strip is complete. The test takes 10 min, and it can be performed either directly on stool or on MacConkey broth culture. For the direct test the stool is suspended in diluent supplied by the manufacturer and placed in the test well. For the broth culture test, stool is inoculated into a MacConkey broth and incubated overnight, and the test performed by placing the broth culture in the sample well.
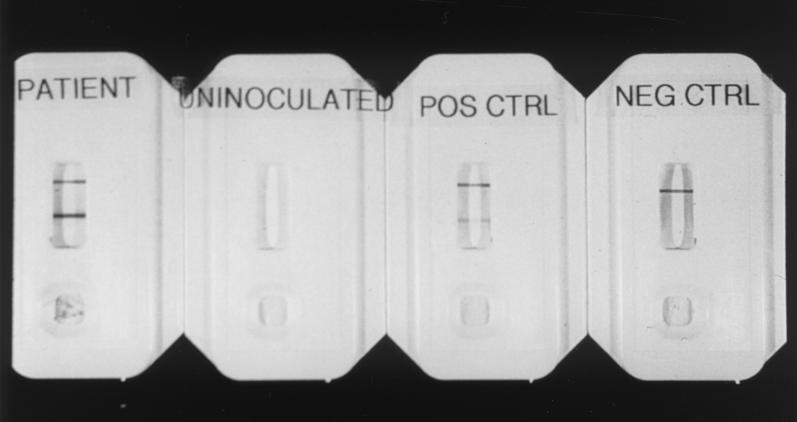
FIG. 1.
From left to right, images show a positive result obtained with a patient specimen (two bands), an uninoculated card, a positive control with two bands, and a negative control with a single band demonstrating validity of test (for explanation, see Materials and Methods).
Study design.
Two studies were performed, a prospective clinical study in five centers, and a retrospective study at three centers using stored frozen stool specimens. In the clinical study both the direct test and the incubation test were performed. For the direct test stools received without transport medium were diluted 1:15 in the manufacturer's diluent and specimens in Cary-Blair transport medium were diluted 1:4. For the incubation test a portion of the stool was cultured overnight in MacConkey broth and the test was performed directly on the broth after incubation (dilution, 1:15).
The “gold standard” for evaluation of ICS was culture of E. coli O157:H7. In each of the five laboratories participating in the clinical studies, stools were cultured for E. coli O157:H7 on sorbitol-MacConkey agar and sorbitol-negative colonies were identified as O157 by latex agglutination. H7 was identified by agglutination (four laboratories) or flagellar immobilization (one laboratory).
For the retrospective study the direct test only was performed as described above. A total of 417 stored stool specimens, culture positive for E. coli O157:H7, from the Laboratory Centre for Disease Control and from Fairfax Hospital were tested (after thawing) with the direct ICS test according to the manufacturer's instructions. Negative controls (103 specimens) included 50 specimens with alternate pathogens, as follows: Campylobacter sp., 26 specimens; Salmonella sp., 9 specimens; Shigella sp., 3 specimens; non-O157 VTEC, 12 specimens). All specimens had been frozen for up to 9 years at −20°C.
RESULTS
The results of the clinical study are shown in Table 1. Twelve of 14 culture-positive stool specimens were positive by both tests. One specimen was positive after incubation but negative by the direct test, and this specimen yielded a single colony of E. coli O157:H7 after an exhaustive search, in excess of normal clinical routine. One was positive by the direct test but negative by the incubation test. This specimen also yielded the organism after an augmented search. A total of 14 of 14 stool specimens were positive in at least one of the tests (direct or after incubation). There were no false positives among 263 culture-negative specimens.
TABLE 1.
Performance of ICS test in prospective clinical study
| Culture | No. of specimens positive/no. tested
|
|||
|---|---|---|---|---|
| D-ICSa | I-ICSb | Bothc | Either test | |
| Positive | 13/14 | 13/14 | 12/14 | 14/14 |
| Negative | 0/263 | 0/263 | 0/263 | 0/263 |
D-ICS, ICS direct test.
I-ICS, ICS test after overnight incubation in broth.
Data indicate the number of specimens whose results were positive by ICS direct test and by ICS test after overnight incubation in broth.
Data indicate the number of specimens whose results were positive either by ICS direct test, by ICS test after overnight incubation in broth or by both.
Table 2 shows the results of the retrospective study. The test results were positive for 339 (81%) of 417 culture-positive specimens, and the specificity was 95% 98 of 103 specimens. Of the 339 test-positive specimens, 328 had been frozen without preservation and 11 had been preserved in Cary-Blair medium. The day of illness on which the specimen was taken was available for 361 of the 417 culture-positive specimens in the retrospective study. The direct test result was positive for 85% (269 of 317 specimens) of stool samples taken on or before the fifth day of illness and for 73% (32 of 44 specimens) stool samples taken on the sixth day or later.
TABLE 2.
Performance of direct ICS test in retrospective study using stored specimens
| ICS test result | No. of specimens whose result by culturec was:
|
|||
|---|---|---|---|---|
| Positive
|
Negative
|
|||
| No CBa | In CBb | No CB | In CB | |
| Positive (no. of specimens) | 328 | 11 | 5 | 0 |
| Negative (no. of specimens) | 74 | 4 | 77 | 21 |
| Sensitivity (%)d | 82 | 77 | NAe | NA |
| Specificity (%) | NA | NA | 94 | 100 |
Specimens frozen without transport medium (Cary-Blair [CB] medium).
Specimens preserved in Cary-Blair (CB) medium.
E. coli O157:H7 culture.
No significant difference between values for culture-positive specimens (P > 0.05, χ2 test).
NA, not applicable.
DISCUSSION
In the clinical study the ICS test result was positive for 14 of 14 culture-positive stools (Table 1). This number suggests a high sensitivity, when considered with the overall 81% sensitivity demonstrated in the retrospective study, which is probably an underestimate (see below and Table 2). In the clinical specimen for which the result was negative by the direct test but positive after incubation, it is likely that initially the number of organisms was too small to be detected and that the incubation step enhanced the population of E. coli O157:H7 over the detection threshold. Antibiotic in the specimen might explain the result on the specimen which was positive by the direct test but negative after incubation, although we were not able to verify this. The ICS test requires the presence of both antigens (O157 and H7), and the capture system requires the structural integrity of the organisms, to ensure the comigration of the two antigens along the membrane. We suggest that the organisms were killed and disrupted by incubation with antibiotic, although enough bacteria were present in the fresh specimen to yield a positive direct test result. This requirement for the presence of two separate antigens on intact organisms to yield a positive result is probably also the explanation for the specificity of the test. Both of the apparent false-positive results were resolved by an enhanced search for viable organisms, suggesting that the sensitivity of this test for this patient population is comparable to that of standard culture. The specificity of ICS in the clinical study was 100% (263 of 263 specimens).
The sensitivity of the direct test performed on the stored specimens was 85% for specimens taken on or before the fifth day of illness. The sensitivity of the test is probably dependent on the number of organisms present in the specimen submitted; it is likely that storage conditions had an impact on the number of organisms. Therefore, the overall sensitivity of 81% on frozen and thawed specimens in the retrospective study would underestimate the sensitivity of the direct test in clinical use. It has been demonstrated in cases of VTEC diarrhea that the number of organisms in the stool decreases progressively after the first few days of infection (8). This may be the reason for the lower sensitivity of the test in specimens taken after the fifth day of the illness. The number of specimens in Cary-Blair medium was not large enough to demonstrate a total absence of effect due to this medium, but the numbers in Table 2 do demonstrate that any effect due to Cary-Blair medium is not great. The absence of false-positive results on the 50 specimens from which alternate pathogens were isolated is reassuring, particularly in the case of Campylobacter sp. infections which, like VTEC, frequently present with bloody diarrhea.
Commercially available rapid tests for VTEC include those which detect verotoxin in stools or culture supernatants and those which detect structural bacterial antigen (4, 5, 7, 9). ICS is more sensitive and specific than the Premier E. coli O157 test previously reported (7).
The ICS test is simple to perform, the direct test gives a result within 10 min, and the test will be of particular value in areas where E. coli O157:H7 is the predominant VTEC serotype. Early detection of patients at risk will permit closer observation for signs of the onset of hemolytic-uremic syndrome, and rapid laboratory diagnosis also facilitates management of outbreaks.
ACKNOWLEDGMENTS
We acknowledge the help of the following, who supplied the specimens used in the retrospective study: Nevio Cimolai, British Columbia Children's Hospital, Vancouver, British Columbia, Canada; Chandar Anand, Provincial Laboratory of Public Health for Southern Alberta, Calgary, Alberta, Canada; Robert Rennie, University of Alberta Hospital, Edmonton, Alberta, Canada; Kathy Manarin, Victoria Hospital Corporation, London, Ontario, Canada; Fiona Smaill, McMaster University Medical Centre, Hamilton, Ontario, Canada; Colina Jones, St. Joseph's Hospital, Hamilton, Ontario, Canada; Mohammed Karmali, The Hospital for Sick Children, Toronto, Ontario, Canada; Gloria Delisle, Hotel Dieu Hospital, Kingston, Ontario, Canada; Silvana Trifiro, Montreal Children's Hospital, Montreal, Quebec, Canada; Lucette Lafleur, L'Hôpital Sainte Justine, Montreal, Québec, Canada; Louise Coté, Centre Hôpitalier de L'University de Québec, St. Foy, Québec, Canada; George Nelson, Isaak Walton Killam Hospital for Children, Halifax, Nova Scotia, Canada; and Jim Flemming, Sir Charles A. Janeway Child Health Centre, St. Johns, Newfoundland, Canada.
This study was funded by Meridian Diagnostics Inc.
REFERENCES
- 1.Brotman M, Giannella R A, Alm P F, Bauman H, Bennett A R, Black R E, Bruhn C M, Cohen M B, Gorbach S L, Kaper J B, Roberts M R, Staneck J L, Taylor S, Troutt H F, Bell B P, Buchanan R L, Durham K, Feng P, Forman C T, Galler R G, Gravani R B, Hall R B, Hancock D D, Hollingsworth J, Karmali M A, et al. Consensus conference statement—Escherichia coli O157:H7 infections—an emerging national health crisis, July 11–13, 1994. Gastroenterology. 1995;108:1923–1934. [PubMed] [Google Scholar]
- 2.Griffin P, Tauxe R. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 3.Griffin P M. Epidemiology of Shiga toxin-producing Escherichia coli in humans in the United States. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C.: American Society for Microbiology; 1998. pp. 15–22. [Google Scholar]
- 4.Karmali M A, Petric M, Bielaszewska M. Evaluation of a microplate latex agglutination method (Verotox-F assay) for detecting and characterizing verotoxins (Shiga toxins) in Escherichia coli. J Clin Microbiol. 1999;37:396–399. doi: 10.1128/jcm.37.2.396-399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehl K S, Havens P, Behnke C E, Acheson D W K. Evaluation of the Premier EHEC assay for detection of Shiga toxin-producing Escherichia coli. J Clin Microbiol. 1997;35:2051–2054. doi: 10.1128/jcm.35.8.2051-2054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loirat C, Sonsino E, Moreno M, Pillion G, Mercier J C, Beaufils F, Mathieu H. Hemolytic-uremic syndrome: an analysis of the natural history and prognostic features. Acta Paediatr Scand. 1984;73:505–514. doi: 10.1111/j.1651-2227.1984.tb09962.x. [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie A M R, Lebel P, Orrbine E, Rowe P C, Hyde L, Chan F, Johnson W, McLaine P N The Synsorb PK Study Investigators. Sensitivities and specificities of Premier E. coli O157 and Premier EHEC Enzyme Immunoassays for diagnosis of infection with verotoxin (Shiga-like toxin)-producing Escherichia coli. J Clin Microbiol. 1998;36:1608–1611. doi: 10.1128/jcm.36.6.1608-1611.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pai C H, Gordon R, Sims H V, Brian L E. Sporadic cases of hemorrhagic colitis associated with Escherichia coli O157:H7. Ann Int Med. 1984;101:738–742. doi: 10.7326/0003-4819-101-6-738. [DOI] [PubMed] [Google Scholar]
- 9.Park C H, Gates K M, Vandel N M, Hixon D L. Isolation of Shiga-like toxin producing Escherichia coli (O157 and non-O157) in a community hospital. Diagn Microbiol Infect Dis. 1996;26:69–72. doi: 10.1016/s0732-8893(96)00180-0. [DOI] [PubMed] [Google Scholar]
- 10.Rowe P C, Orrbine E, Lior H, Wells G A, Yetisir E, Clulow M, McLaine P N Investigators of the Canadian Pediatric Kidney Disease Research Center. Risk of hemolytic uremic syndrome after sporadic Escherichia coli O157:H7 infection: results of a Canadian collaborative study. J Pediatr. 1998;132:777–781. doi: 10.1016/s0022-3476(98)70303-8. [DOI] [PubMed] [Google Scholar]
- 11.Van Dyck M, Proesmans W C, Depraetere M. Hemolytic uremic syndrome in childhood: renal function ten years later. Clin Nephrol. 1988;29:109–112. [PubMed] [Google Scholar]