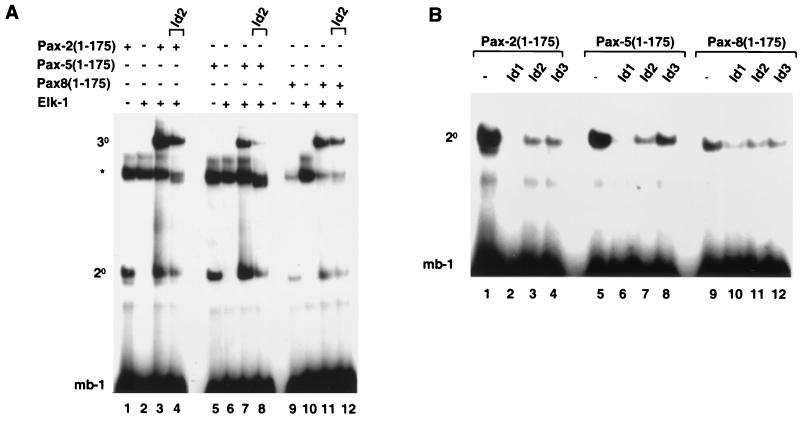
FIG. 3.
Id proteins inhibit nucleoprotein complex formation by all members of the Pax-2/-5/-8 subfamily of Pax proteins. Shown is a gel retardation analysis of complex formation by Pax-2, Pax-5, and Pax-8 on the mb-1 promoter in the presence of Elk-1 and/or Id1, Id2, or Id3. The locations of binary Pax-DNA (2°) and ternary Pax–Elk-1–DNA (3°) complexes and the position of a complex arising from the reticulocyte lysate (asterisk) are indicated. (A) Complex formation by in vitro-translated Pax-2(1-175) (lanes 1 to 4), Pax-5(1-175) (lanes 5 to 8), and Pax-8(1-175) (lanes 9 to 12) and full-length recombinant Elk-1 (lanes 2, 3, 4, 6, 7, 8, 10, 11, and 12) and the mb-1 site. Equal molar ratios of each Pax protein were used. In vitro-translated Id2 was added to the binding reaction mixtures in lanes 4, 8, and 12. (B) Binary complex formation by Pax-2(1-175) (lanes 1 to 4), Pax-5(1-175) (lanes 5 to 8), and Pax-8(1-175) (lanes 9 to 12) in the presence of equimolar amounts (with respect to each Id protein) of in vitro-translated Id1 (lanes 2, 6, and 10), Id2 (lanes 3, 7, and 11), and Id3 (lanes 4, 8, and 12). For all reactions the volumes of reticulocyte lysate added to each lane were equalized by adding appropriate volumes of unprogrammed lysate.