Abstract
Coronavirus disease 2019 (COVID‐19) is characterized by dysregulated hyperimmune response and steroids have been shown to decrease mortality. However, whether higher dosing of steroids results in better outcomes has been debated. This was a retrospective observation of COVID‐19 admissions between March 1, 2020, and March 10, 2021. Adult patients (≥18 years) who received more than 10 mg daily methylprednisolone equivalent dosing (MED) within the first 14 days were included. We excluded patients who were discharged or died within 7 days of admission. We compared the standard dose of steroids (<40 mg MED) versus the high dose of steroids (>40 mg MED). Inverse probability weighted regression adjustment (IPWRA) was used to examine whether higher dose steroids resulted in improved outcomes. The outcomes studied were in‐hospital mortality, rate of acute kidney injury (AKI) requiring hemodialysis, invasive mechanical ventilation (IMV), hospital‐associated infections (HAI), and readmissions. Of the 1379 patients meeting study criteria, 506 received less than 40 mg of MED (median dose 30 mg MED) and 873 received more than or equal to 40 mg of MED (median dose 78 mg MED). Unadjusted in‐hospital mortality was higher in patients who received high‐dose corticosteroids (40.7% vs. 18.6%, p < 0.001). On IPWRA, the use of high‐dose corticosteroids was associated with higher odds of death (odds ratio [OR] 2.14; 95% confidence interval [CI] 1.45–3.14, p < 0.001) but not with the development of HAI, readmissions, or requirement of IMV. High‐dose corticosteroids were associated with lower rates of AKI requiring hemodialysis (OR 0.33; 95% CI 0.18–0.63). In COVID‐19, corticosteroids more than or equal to 40 mg MED were associated with higher in‐hospital mortality.
Keywords: COVID‐19, corticosteroids, outcomes
Highlights
In this observational study of 1379 patients, using Inverse probability weighted regression adjustment, those receiving 30 mg (IQR 24‐34 mg) methylprednisolone equivalent dose (MED) had lower in hospital mortality than those with 78 mg (IQR 59‐108 mg) MED.
Higher dose steroids were not associated with better improvements in inflammatory markers, rates or duration of mechanical ventilation or readmissions.
Higher dose steroids were however, associated with lower rates of acute kidney injury requiring hemodialysis.
Dosage higher than 80 mg MED were associated with higher rates of hospital acquired infections.
Abbreviations
- AKI
acute kidney injury
- AWS
Amazon Web Services
- CI
confidence Interval
- CPT
current procedural terminology
- CRP
C reactive peptide
- HAI
hospital acquired infection.
- ICD10CM
International classification of disease 10th Clinical Modification
- ICU
intensive care unit
- IMV
invasive mechanical ventilation
- IPRWA
inverse probability weighted regression adjustment
- LDH
lactate dehydrogenase
- LOS
length of hospital stay
- LTAC
long‐term acute care
- OR
odds ratio
- SNF
skilled nursing facility
- SOFA
Sequential Organ Failure Assessment
- VTE
venous thromboembolism
1. INTRODUCTION
Recently, the Society of Critical Care Medicine has made a strong recommendation to use a short course of systemic corticosteroids. 1 This was based on metanalysis by the REACT working group, which showed a significant reduction in 28‐day mortality in patients receiving steroids. 2 The reduction in mortality was observed for dexamethasone and hydrocortisone treatments. The RECOVERY trial comprised the largest study in the metanalysis and used dexamethasone 6 mg daily for 10 days. 3 At the point of writing this manuscript, steroids have been shown to be useful in moderate to severe coronavirus disease 2019 (COVID‐19)—those requiring high flow oxygen and those requiring noninvasive or invasive mechanical ventilation (IMV). The dose of the steroids has been debated.
Researchers have observed dose‐dependent activation of corticosteroid receptors without an increase in adverse effects. 4 Whether this effect results in improved outcomes is currently unknown. A few randomized controlled trials (RCTs) used high‐dose methylprednisolone for short periods 5 , 6 , 7 , 8 and have reported conflicting results. Some researchers have proposed a higher dose of steroids, including pulse dosing with good results. 9 , 10 In a nonviral acute respiratory distress syndrome (ARDS), high dose steroids followed by tapering dose was shown to improve mortality and ventilator‐free days without increased risk of serious side effects. 11 In other viral illnesses such as the Middle East respiratory syndrome, high dose steroids were beneficial in severe cases while in H1N1 pneumonia, mild to moderate dose improved outcomes, and higher dose did not. 12 , 13 In an observational study of 447 COVID‐19 patients, high dose steroids improved mortality and progression to mechanical ventilation. 9 However, the optimal dose of corticosteroids in COVID‐19 is still unknown. RCTs are ongoing to answer this question. 14 , 15
The objectives of this retrospective study were to determine if a higher dose of corticosteroids was associated with improvement in outcomes of hospitalized COVID‐19 patients. We used inverse probability weighted regression adjustment, a statistical method that has been shown to minimize bias among propensity score methods. 16
2. METHODS
2.1. Study design and data source
We performed a retrospective analysis of adult COVID‐19 patients (age ≥ 18 years) admitted to a large community hospital in a rural setting in Northeast Georgia between March 1, 2020, and March 10, 2021. COVID‐19 patients were identified from our Epic® EMR using ICD10CM and/or CPT codes for COVID‐19 infection and/or positive COVID‐19 testing. We obtained clinical and demographical details of patients from Epic® Caboodle data warehouse and Cerner APACHE® Outcomes. Systems integration was provided by IPC Global; Atlanta and we leveraged their in‐Process Data Factory innovation running on an AWS® VPC. Readmissions of the patients with COVID‐19 patients were excluded. The study was reviewed and found exempt by the Institutional Review Board of the Northeast Georgia Health System.
2.2. Definitions
We obtained the type and dose of corticosteroids used for the COVID19 patient. The selection method for the study is shown in Figure S1. We excluded those who did not receive any corticosteroids. We also excluded those patients who either were discharged within 7 days of admission or died within 7 days of admission to exclude nonsick and extremely sick patients who would have either survived or died with or without corticosteroids. We used methylprednisolone equivalent dosing (MED) for comparison. 1 mg of dexamethasone was equivalent to 5 mg of methylprednisolone. We calculated the total amount of MED received within the first 14 days of admission. An average daily dose of steroids was calculated by dividing the total dose received in the first 14 days by the total number of days of corticosteroids were received within the first 14 days. We excluded patients whose daily average dose was less than 10 mg and those who received the first dose of corticosteroids after 14 days of admission.
We used restricted cubic splines to examine the probability of death with MED dose to determine the cutoff values of high‐dose corticosteroids. The graph showed two elbows (Figure S2). The lower elbow was at about 40 mg MED and the upper elbow was at 80 mg MED. As the standard dosing in COVID‐19 studies involved 6 mg of dexamethasone, which is equivalent to about 30 mg of MED, we used 40 mg MED as our cutoff. Thus, we divided steroid dosing into two groups—(1) standard dose (if they received < 40 mg MED daily dose) and (2) high dose (if they received ≥ 40 mg MED daily dose).
We collected clinical and demographic data including comorbidities, home anticoagulation, antiplatelet use, laboratory values, inflammatory markers (ferritin, C‐reactive peptide [CRP], lactate dehydrogenase [LDH], fibrinogen, and d‐dimer), the presence of central venous catheters, and medications given for COVID‐19 infection (remedesivir, corticosteroids, tocilizumab, convalescent plasma, hydroxychloroquine, and ivermectin). We collected data for hospital‐acquired infections (HAI), IMV acute kidney injury (AKI), acute kidney injury requiring hemodialysis, venous thromboembolism (VTE), acute strokes, blood transfusions, and pneumothorax. The severity of illness was defined by Sequential Organ Failure Assessment (SOFA) score and 4C score. 17
2.3. Definition of variables
Our primary dependent variable was in‐hospital mortality. Other outcomes studied were the rate of AKI requiring hemodialysis, IMV, HAI, readmission, and duration of IMV.
2.4. Change in inflammatory markers
We obtained the highest inflammatory marker level within the first 5 days of admission and compared it with the lowest inflammatory marker during the subsequent 3 weeks. We only compared those patients who had both sets of values. We compared changes in the level of ferritin, CRP, LDH, and fibrinogen in standard and high dose steroid groups using t‐test (after log transformation of the inflammatory markers) and Wilcoxon rank test for d‐dimer.
2.5. Statistics
We described the categorical data using frequency count and percentages. We report means and standard deviation or medians and interquartile ranges (IQRs) for continuous variables as appropriate for their distribution. We compared demographical and clinical characteristics using the χ 2 test and the Wilcoxon rank test for categorical and continuous variables, respectively. For all analyses, we deemed statistical significance a p‐value less than 0.05.
2.6. Model to examine treatment effect of high dose corticosteroids
To compare the two regimens of corticosteroids (high dose vs. standard dose), we used inverse probability weighted regression adjustment (IPWRA) to correct for potential bias brought about by higher doses of steroids in sicker patients. We calculated the probability of receiving high dose corticosteroids (propensity score model) by fitting a multivariable logistic regression model with high dose steroid as the dependent variable and patient demographics, comorbidities, use of anticoagulants and antiplatelet agents before hospitalization, SOFA score, and 4C score on admission, IMV, intensive care unit (ICU) transfer, use of vasopressors, medications related to COVID‐19 (remedesivir, ivermectin, hydroxychloroquine, tocilizumab, ad convalescent plasma), and initial inflammatory markers (ferritin, d‐dimer, fibrinogen, LDH, and CRP). These predictors were chosen based on clinical judgment and model fit. We log‐transformed (natural logs) markers of inflammation as they were not normally distributed. For d‐dimer, we used deciles as we were unable to normalize its distribution. We imputed missing values of inflammatory markers using median values. Inverse probability weights were obtained by using an inverse of the predicted probability of anticoagulation from the propensity score model. Next, we fitted our outcome model (in‐hospital mortality) using the inverse probability weights and adjusted this regression model for patient demographics, comorbidities, SOFA and 4C score, IMV, ICU transfer, use of vasopressors, COVID‐19 medications received, initial inflammatory markers (ferritin, d‐dimer, fibrinogen, LDH, and CRP) and complications such as VTE, acute stroke, AKI requiring hemodialysis, HAI, pneumothorax, and blood transfusion.
We used a similar approach for other outcomes such as AKI requiring hemodialysis, mechanical ventilation, hospital‐acquired infections, and rates of readmission. For our secondary analysis, we excluded patients who developed respective outcomes before initiation of corticosteroids. For example, for analysis of the risk of IMV, we excluded patients who received corticosteroids after they were intubated.
As an exploratory analysis, we compared a daily dose of MED more than or equal to 80 mg per day with those who received less than 80 mg per day to examine if an extremely high dose of corticosteroids were associated with reduced in‐hospital mortality and other secondary outcomes. We used similar statistical methods as described above. We performed all statistical analyses using STATA MP 16.0 (Stata‐Corp).
3. RESULTS
There were 5204 COVID‐19 admissions during the study period. Of these 1379 met inclusion criteria (Figure 1). Of these, 506 received less than 40 mg of MED and 873 received more than or equal to 40 mg of MED. The median dose of corticosteroids in patients receiving the standard dose of steroid was 30 mg MED (IQR 24–34 mg) and 78 mg MED (IQR 59–108 mg) in the high dose group. The median time to initiation of corticosteroids was 0.42 days in the standard dose and 0.18 days in the high dose (Table 1).
Figure 1.
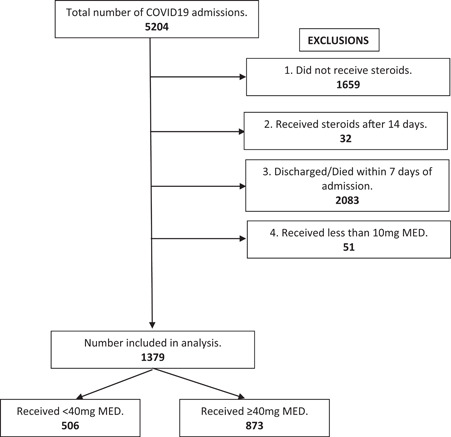
Study design and definitions of steroids. MED is methylprednisolone equivalent dose (1 mg dexamethasone = 5 mg methylprednisolone). Rationale for 1—did not receive steroids. Rationale for 2—delayed steroid treatment. Rationale for 3—got better too quickly or died too quickly. Rationale for 4—received too little of a dose. COVID‐19, coronavirus disease 2019
Table 1.
Demographical, clinical characteristics, and outcomes of COVID‐19 patients receiving steroids
| Standard dose | High dose | p value | |
|---|---|---|---|
| Total | 506 | 873 | |
| Daily MED in mg, median (IQR) | 30 (24–34) | 78 (59–108) | |
| Time to steroids in days, median (IQR) | 0.42 (0.14–1.8) | 0.18 (0.05–0.57) | <0.001 |
| Age, median (IQR)* | 73 (60–81) | 69 (59–76) | 0.001 |
| Male (%) | 55.9 | 59.6 | 0.18 |
| Race (%)* | 0.71 | ||
| White | 73.9 | 72.3 | |
| Blacks | 8.9 | 8.0 | |
| Hispanic | 12.4 | 14.4 | |
| Asian | 1.6 | 2.3 | |
| Not disclosed | 3.2 | 3.0 | |
| Blood group A (%) | 36 | 35.2 | 0.91 |
| BMI, median (IQR) | 29 (25–35) | 31 (27–37) | 0.56 |
| Comorbidities (%) | |||
| Hypertension | 80.8 | 79.0 | 0.42 |
| Congestive heart failure | 39.9 | 36.7 | 0.22 |
| Diabetes mellitus | 54.9 | 54.8 | 0.94 |
| Chronic obstructive pulmonary disease | 41.9 | 44.1 | 0.42 |
| End‐stage renal disease | 6.1 | 5.2 | 0.44 |
| Cirrhosis | 10.3 | 13.1 | 0.12 |
| Cancer | 15.0 | 14.4 | 0.76 |
| History of VTE | 6.9 | 7.2 | 0.83 |
| Alcoholism | 2.8 | 2.5 | 0.68 |
| Rheumatological disease | 3.8 | 5.2 | 0.22 |
| Home meds (%) | |||
| Anticoagulation | 16.4 | 10.0 | 0.001 |
| NSAIDs | 15.2 | 17.5 | 0.26 |
| COVID‐19 medications (%) | |||
| Tocilizumab | 10.9 | 16.6 | 0.004 |
| Remedesivir | 75.1 | 82.4 | 0.001 |
| Anticoagulation – High dose* | 39.5 | 51.7 | 0.001 |
| SOFA score at admission | 1 (0–2) | 1 (0–2) | 0.06 |
| 4C score at admission | 13 (10–15) | 12 (9–14) | 0.31 |
Abbreviations: BMI, body mass index; COVID‐19, coronavirus disease 2019; IQR, interquartile range; MED, methylprednisolone equivalent dosing; NSAIDs, non‐steroidal anti‐inflammatory drugs; SOFA, Sequential Organ Failure Assessment; VTE, venous thromboembolism.
>1 mg/kg of enoxaparin a day or 7500 TID of heparin or higher.
3.1. Characteristics of patients receiving high‐dose corticosteroids
Patients who received high‐dose corticosteroids were younger but other demographical and comorbidities were similar in both steroid groups (Table 1). Patients who received high‐dose corticosteroids more often received other possible therapies for COVID‐19 (tocilizumab, remdesivir, and higher dose anticoagulation). The severity of illness as observed by SOFA score and 4C score were not statistically different on admission between the two groups (Table 1).
A patient who received high dose corticosteroids were/became sicker than their counterparts as adjudicated by higher rates of ICU admissions (67.8% vs. 42.3%, p < 0.001), ventilator use (28.1% vs. 11%, p < 0.001), lower PF ratio on a ventilator (52 vs. 58, p = 0.006), and vasopressor requirements (14.7% vs. 9%, p = 0.003) (Table 2). Also, In the cohort that received high dose corticosteroids, both AKI and AKI requiring hemodialysis, HAIs (including candidemia), VTE, and blood transfusions were more common than in the cohort receiving standard‐dose corticosteroids (Table 3).
Table 2.
ICU characteristics of COVID‐19 patients receiving steroids
| Low dose | High dose | p value | |
|---|---|---|---|
| ICU, N (%) | 214 (42.3) | 592 (67.8) | 0.001 |
| SOFA score at ICU admission | 2 (0–4) | 1 (0–3) | 0.01 |
| Ventilator use * N (%) | 50 (11) | 201 (28.1) | 0.001 |
| Length of mechanical ventilation*, days | 7.5 (2–13) | 9 (4–17) | 0.06 |
| Lowest PF ratio | 58 (49–73) | 52 (45–62) | 0.006 |
| Required paralysis (%) | 32 | 47.7 | 0.045 |
| Inhaled vasodilators (%) | 8 | 15.9 | 0.15 |
| Tracheostomy (%) | 12 | 17.4 | 0.35 |
| Use of vasopressors | |||
| Required norepinephrine (%) | 39.3 | 57.4 | 0.001 |
| Required vasopressin (%) | 21 | 31.6 | 0.003 |
| Required epinephrine (%) | 9.4 | 12.6 | 0.09 |
| Required dobutamine (%) | 4.7 | 6.6 | 0.30 |
| Central venous catheters | 27.1 | 45.6 | 0.001 |
Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment;.
if received steroids > 2 days before intubation.
Table 3.
Outcomes of COVID‐19 patients receiving steroids
| Low dose | High dose | p value | |
|---|---|---|---|
| Complications (%) | |||
| Acute stroke | 3.2 | 5.4 | 0.057 |
| Acute intracranial bleeding | 1.8 | 2.4 | 0.44 |
| Acute kidney injury | 25.1 | 36.5 | 0.001 |
| Acute renal failure requiring hemodialysis | 5.1 | 7.8 | 0.06 |
| Venous thromboembolism | 4.6 | 10.6 | 0.001 |
| Blood transfusion | 13 | 25.2 | 0.001 |
| Hospital‐acquired infections | 6.1 | 15.2 | 0.001 |
| Candidemia | 1.2 | 3.6 | 0.009 |
| In‐hospital mortality (%) | 18.6 | 40.7 | 0.001 |
| 28‐day mortality (%) | 17.2 | 39.8 | 0.001 |
| LOS in survivors, median (IQR) | 12 (9–18) | 14 (10–23) | 0.001 |
| Time to death, median (IQR) | 15 (11–24) | 18 (12–26) | 0.13 |
| Disposition (%) | 0.034 | ||
| Home | 36.1 | 39.0 | |
| Home with health | 28.8 | 31.4 | |
| SNF/LTAC/Rehab | 31.4 | 24.8 | |
| Others | 3.7 | 4.9 | |
| Readmissions (%) | 18.9 | 15.1 | 0.11 |
| Death during readmission (%) | 26.4 | 30.7 | 0.51 |
Abbreviations: COVID‐19, coronavirus disease 2019; IQR, interquartile range; LOS, length of hospital stay; LTAC, long‐term acute care; SNF, skilled nursing facility.
Table 4.
Association of high dose steroids (≥40 mg MED) with primary and secondary outcomes using IPRWA modeling
| Outcome | Odds ratio | 95%CI | p value |
|---|---|---|---|
| In‐hospital mortality | 2.14 | 1.45–3.13 | 0.001 |
| Hospital associated infections | 1.00 | 0.59–1.68 | 0.99 |
| Candidemia | 0.51 | 0.13–1.99 | 0.34 |
| AKI requiring hemodialysis | 0.34 | 0.18–0.63 | 0.001 |
| Invasive mechanical ventilation | 0.77 | 0.40–1.46 | 0.42 |
| Length of mechanical ventilation | 0.18 | −0.07 to 0.42 | 0.16 |
| Readmission | 1.14 | 0.74–1.76 | 0.55 |
Abbreviations: CI, confidence interval; IPRWA, inverse probability weighted regression adjustment; MED, methylprednisolone equivalent dosing.
Unadjusted in‐hospital mortality was higher in patients who received high‐dose corticosteroids (40.7% vs. 18.6%, p < 0.001). However, the rate of discharges to home and with home health was significantly higher in patients who received high‐dose corticosteroids. Readmission rates (15.1% vs. 18.9%, p = 0.11) and death during the readmissions (30.7% vs. 26.4%, p = 0.51) were not significantly different in the two groups.
3.2. Inflammatory markers
Patients who received higher dose corticosteroids, with exception of d‐dimer, had higher levels of ferritin, CRP, LDH, and fibrinogen (Table S2). The improvement in levels of inflammatory markers over 3 weeks was not different between the two groups (Figure S2).
3.3. Standard dose versus high dose (≥40 mg MED) corticosteroids—in‐hospital mortality and other outcomes
Use of high‐dose corticosteroids was associated with higher odds of death (odds ratio [OR] 2.14; 95% confidence interval [CI] 1.45–3.14, p < 0.001). However, there was no significant association of high‐dose corticosteroids with the development of HAI (OR 1.00; 95% CI 0.59–1.68), candidemia (OR 0.51; 95% CI 0.13–2.0, p = 0.33), readmissions (OR 1.14; 95% CI 0.74–1.76, p = 0.55), or requirement or duration of IMV (OR 0.76; 95% CI 0.40–1.46, p = 0.42). However, high dose corticosteroids were associated with lower rates of AKI requiring hemodialysis (OR 0.33; 95% CI 0.18–0.63) (for details see Table S3).
3.4. Effect of ≥ 80 mg MED daily of methylprednisolone
Of the 1379 patients, 414 received more than or equal to 80 mg of MED of corticosteroids. The median dose in this group was 110 mg (IQR 92–130 mg) per day. This was compared to 965 patients who received a median dose of 39 mg MED (IQR 30–60 mg). In‐hospital mortality was still higher in this high steroid group (44.4% vs. 27.5%, p < 0.001). On adjusted analysis, in‐hospital mortality was significantly higher in higher dose corticosteroids (OR 1.63; 95% CI 1.12–2.37, p = 0.01). Higher doses of corticosteroids were also associated with increased risk of HAI (OR 1.70; 95% CI 1.12–2.59, p = 0.014) but not with decreased rates of mechanical ventilation, duration of mechanical ventilation, AKI requiring hemodialysis, and readmissions.
4. DISCUSSION
In this retrospective study, we report that corticosteroids at more than or equal to 40 mg MED were associated with higher in‐hospital mortality after adjusting for multiple covariates including severity. A dose more than or equal to 40 mg MED did not decrease the rate or duration of mechanical ventilation. Similarly, dosages more than or equal to 80 mg MED were also associated with higher in‐hospital mortality and did not improve rates or duration of mechanical ventilation. Dosage more than or equal to 80 mg MED was also associated with higher rates of HAIs. The use of high‐dose corticosteroids did not improve readmissions. To our knowledge, this is the largest retrospective observational study examining this question. This study is designed to be hypothesis‐generating and providing data for future RCTs. We have attempted to provide answers for multiple outcomes of interest.
We started using corticosteroids universally for our patients who required oxygen after initial reports from the RECOVERY trial. We have only used methylprednisolone and dexamethasone in COVID‐19 patients. As there was no consensus over the dosing regimens, we left the decision to treating physicians. The proportion of patients receiving the higher dose of corticosteroids did not change through the study period (Figure S2).
Corticosteroids in ARDS have been subjected to several RCTs. 11 , 18 , 19 , 20 In COVID‐19, there is sufficient data to recommend its use in patients requiring oxygen and those on IMV. However, the answer to optimal steroid dosing is still elusive. The higher dose of corticosteroids has been used in a few RCTs and the outcomes have been mixed. CoDEX trial used 20 mg of dexamethasone (approximately 100 mg of methylprednisolone) for 5 days followed by 10 mg for 5 days or until ICU discharge and found a decrease in ventilator‐free days but not mortality. 7 Using the same regimen, Jamaati et al. 8 did not find any differences in outcomes. In a smaller RCT, pulse dose steroid (250 mg × 3 days) was shown to improve mortality. 5 , 21 In propensity‐matched observational studies, there are reports of improved survival 22 but not by others. 23 , 24 We found significantly higher odds of death with higher dose steroids. With mortality averaging 32.6% in our study, this results in one excess death for every six patients receiving high‐dose steroids.
Lower dose corticosteroids apart from the RECOVERY trial have failed to show any significant differences in mortality. In the METCOVID study, there were no differences in mortality at 28 days when 0.5 mg/kg of methylprednisolone was given twice for 5 days. 6 In the CAPE COVID study, using 200 mg of hydrocortisone for 7 days followed by tapering dose till 14 days did not result in significant differences in death or respiratory support. 25 Similarly, REMAP‐CAP did not show any improvement in mortality, but better odds of organ support‐free days. 26 However, these were smaller studies, and few were stopped early due to preliminary results from the RECOVERY trial.
Both the type and dosage of corticosteroids used in studies for COVID19 have varied widely. The largest trial to date—RECOVERY trial, has used 6 mg of dexamethasone and has reported improved mortality and rates of mechanical ventilation. 3 This dose is equivalent to 20–30 mg of methylprednisolone. Though dexamethasone and methylprednisolone have good glucocorticoid activities, there may be subtle differences between them. Methylprednisolone has been reported to achieve greater concentration in lungs when compared with other corticosteroids. 27 Draghici et al. 28 analyzed changes in gene expressions in COVID‐19 lung to identify drugs to help hyperinflammation and found methylprednisolone to be more efficacious than dexamethasone or hydrocortisone. However, other studies such as molecular docking showed dexamethasone to be better than other glucocorticoids in binding to the active site—GLN498 of ACE2. 29 In another study, hydrocortisone had the strongest effect on ACE2 activation (Xiang). There have been no head‐to‐head RCT comparing different corticosteroids. Thus, we cannot conclude the superiority of one steroid over another. Our assumption of similar beneficial activity among these two corticosteroids may be a limitation while interpreting the results.
Glucocorticoid's mechanism of action is mediated by the glucocorticoid receptor. Glucocorticoid receptor binds with glucocorticoids in the cytoplasm and is then translocated to the nucleus where it inhibits transcription of genes involved in leucocyte and endothelial/epithelial cell activation and subsequent reduction of pro‐inflammatory cytokines, chemokines, and adhesion molecules. We examined changes in inflammatory markers with standard and higher doses of corticosteroids but did not observe significant changes in rates of improvement in the two groups (Appendix). This may suggest that a lower dosage is possibly sufficient to reduce inflammation and a higher dosage may not be needed.
We have reported earlier that corticosteroids are associated with increased risk of HAI in COVID19. 30 In particular, at a higher dose (≥80 mg MED), we found significantly higher rates of HAI in this group. Corticosteroids have been shown to increase rates of readmissions in small studies, but we did not observe this in our patient population. 31 Subsequent mortality during readmission was also similar in the two groups.
The strength of our study is its large size; however, we acknowledge important limitations. First, its single‐center, observational and retrospective nature makes it susceptible to selection bias and prevents us from assigning causation. We attempted to mitigate this bias by including all hospitalized patients with COVID‐19. Although we have attempted to minimize confounders in treatment outcomes through IPRWA modeling, residual confounding may have still can prevent the capture of important unknown factors that affect differences in outcomes. There were about 25% missing values with respect to the inflammatory markers. These may have affected the precision of our estimates. We could not ascertain whether the steroid regimen—both dosing and type, changed during hospitalization depending on HAIs and changing severity of illness, and inflammatory markers.
5. CONCLUSIONS
Corticosteroids more than or equal to 40 mg MED were associated with higher in‐hospital mortality. Higher dosages more than or equal to 80 mg MED were also associated with increased mortality and development of HAI. High‐dose corticosteroids were not associated with decreased rates or duration of mechanical ventilation or readmissions. The use of the standard dose of steroids is suggested till further answers from RCTs are obtained. We suggest adding the risk of venous thromboembolism and acute kidney injury as secondary endpoints.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest.
ETHICS STATEMENT
The study was reviewed and found exempt by the Northeast Georgia Health System IRB board.
AUTHOR CONTRIBUTIONS
Gagan Kumar and Rahul Nanchal: Study design, data analysis, and manuscript writing. Mark Meersman and Drew Dalton: Data validation. Dhaval Patel, Ankit Sakhuja, and Achuta Kumar Guddati: Study design and manuscript writing. Martin Hererra and David Jefferies: Manuscript writing.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
One of the authors (Dr. Ankit Sakhuja) is supported by the National Institute of General Medical Services, 5U54GM104942‐03. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Kumar G, Patel D, Hererra M, et al. Do high‐dose corticosteroids improve outcomes in hospitalized COVID‐19 patients? J Med Virol. 2021;94:372‐379. 10.1002/jmv.27357
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID‐19) in the ICU: first update. Crit Care Med. 2021;49:e219‐e234. [DOI] [PubMed] [Google Scholar]
- 2. The WHO Rapid Evidence Appraisal for COVID‐19 Therapies (REACT) Working Group . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324:1330‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID‐19. N Engl J Med. 2021;384:693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fleishaker DL, Mukherjee A, Whaley FS, Daniel S, Zeiher BG. Safety and pharmacodynamic dose response of short‐term prednisone in healthy adult subjects: a dose ranging, randomized, placebo‐controlled, crossover study. BMC Musculoskelet Disord. 2016;17:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID‐19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID‐19 (Metcovid): a randomised, double‐blind, phase IIb, placebo‐controlled trial. Clin Infect Dis. 2021;72(9):e373‐e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator‐free in patients with moderate or severe acute respiratory distress syndrome and COVID‐19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jamaati H, Hashemian SM, Farzanegan B, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID‐19: a preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897:173947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papamanoli A, Yoo J, Grewal P, et al. High‐dose methylprednisolone in nonintubated patients with severe COVID‐19 pneumonia. Eur J Clin Invest. 2021;51:e13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. López Zúñiga MÁ, Moreno‐Moral A, Ocaña‐Granados A, et al. High‐dose corticosteroid pulse therapy increases the survival rate in COVID‐19 patients at risk of hyper‐inflammatory response. PLoS One. 2021;16:e0243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267‐276. [DOI] [PubMed] [Google Scholar]
- 12. Lee KH, Yoon S, Jeong GH, et al. Efficacy of corticosteroids in patients with SARS, MERS and COVID‐19: a systematic review and meta‐analysis. J Clin Med. 2020;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Long Y, Xu Y, Wang B, et al. Clinical recommendations from an observational study on MERS: glucocorticoids was benefit in treating SARS patients. Int J Clin Exp Med. 2016;9:8865‐8873. [Google Scholar]
- 14. Granholm A, Munch MW, Myatra SN, et al. Higher vs lower doses of dexamethasone in patients with COVID‐19 and severe hypoxia (COVID STEROID 2) trial: protocol for a secondary Bayesian analysis. Acta Anaesthesiol Scand. 2021;65:702‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maláska J, Stašek J, Duška F, et al. Effect of dexamethasone in patients with ARDS and COVID‐19 ‐ prospective, multi‐centre, open‐label, parallel‐group, randomised controlled trial (REMED trial): a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661‐3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta RK, Harrison EM, Ho A, et al. Development and validation of the ISARIC 4C deterioration model for adults hospitalised with COVID‐19: a prospective cohort study. Lancet. Respir Med. 2021;9:349‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meduri GU, Bridges L, Shih MC, Marik PE, Siemieniuk RAC, Kocak M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial‐level meta‐analysis of the updated literature. Intensive Care Med. 2016;42:829‐840. [DOI] [PubMed] [Google Scholar]
- 19. Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671‐1684. [DOI] [PubMed] [Google Scholar]
- 20. Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954‐963. [DOI] [PubMed] [Google Scholar]
- 21. Ruiz‐Irastorza G, Pijoan JI, Bereciartua E, et al. Second week methyl‐prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: an observational comparative study using routine care data. PLoS One. 2020;15:e0239401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tortajada C, Colomer E, Andreu‐Ballester JC, Esparcia A, Oltra C, Flores J. Corticosteroids for COVID‐19 patients requiring oxygen support? Yes, but not for everyone: effect of corticosteroids on mortality and intensive care unit admission in patients with COVID‐19 according to patients' oxygen requirements. J Med Virol. 2021;93:1817‐1823. [DOI] [PubMed] [Google Scholar]
- 23. Rodríguez‐Molinero A, Pérez‐López C, Gálvez‐Barrón C, et al. Association between high‐dose steroid therapy, respiratory function, and time to discharge in patients with COVID‐19: Cohort study. Med Clin (Engl Ed). 2021;156:7‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monreal E, Sainz de la Maza S, Natera‐Villalba E, et al. High versus standard doses of corticosteroids in severe COVID‐19: a retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2021;40:761‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dequin PF, Heming N, Meziani F, et al. Effect of hydrocortisone on 21‐day mortality or respiratory support among critically ill patients with COVID‐19: a randomized clinical trial. JAMA. 2020;324:1298‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Angus DC, Derde L, Al‐Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID‐19: the REMAP‐CAP COVID‐19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greos LS, Vichyanond P, Bloedow DC, et al. Methylprednisolone achieves greater concentrations in the lung than prednisolone. A pharmacokinetic analysis. Am Rev Respir Dis. 1991;144:586‐592. [DOI] [PubMed] [Google Scholar]
- 28. Draghici S, Nguyen TM, Sonna LA, et al. COVID‐19: disease pathways and gene expression changes predict methylprednisolone can improve outcome in severe cases. Bioinformatics. Published online March 9, 2021. [DOI] [PMC free article] [PubMed]
- 29. Zhang Y, Hu S, Wang J, Xue Z, Wang C, Wang N. Dexamethasone inhibits SARS‐CoV‐2 spike pseudotyped virus viropexis by binding to ACE2. Virology. 2021;554:83‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kumar G, Adams A, Hererra M, et al. Predictors and outcomes of healthcare‐associated infections in COVID‐19 patients. Int J Infect Dis. 2021;104:287‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chaudhry Z, Shawe‐Taylor M, Rampling T, et al. Short durations of corticosteroids for hospitalised COVID‐19 patients are associated with a high readmission rate. J Infect. 2021;82:276‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


