Abstract
The disease produced by the severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2) is currently one of the primary concerns worldwide. Knowing the zoonotic origin of the disease and that several animal species, including dogs and cats, are susceptible to viral infection, it is critical to assess the relevance of pets in this pandemic. Here, we performed a large‐scale study on SARS‐CoV‐2 serological and viral prevalence in cats and dogs in Spain in order to elucidate their role and susceptibility. Samples from animals in contact with COVID‐19 positive people and/or compatible symptoms (n = 492), as well as from random animals (n = 1024), were taken. Despite the large number of animals analyzed, only 12 animals (eight dogs and four cats), which represents 0.79% of the total analyzed animals (n = 1516), were positive for viral SARS‐CoV‐2 RNA detection by reverse transcription quantitative PCR (RT‐qPCR) in which viral isolation was possible in four animals. We detected neutralizing antibodies in 34 animals, four of them were also positive for PCR. This study evidences that pets are susceptible to SARS‐CoV‐2 infection in natural conditions but at a low level, as evidenced by the low percentage of positive animals detected, being infected humans the main source of infection. However, the inclusion of animals in the surveillance of COVID‐19 is still recommended.
Keywords: cats, dogs, neutralizing antibodies, RT‐qPCR, SARS‐CoV‐2, viral isolation
1. INTRODUCTION
The first case of severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2) was reported in Wuhan, Hubei province, China, in December 2019 (Zhou et al., 2020). The virus has spread worldwide, leading to one of the most challenging pandemics to date (Abdel‐Moneim & Abdelwhab, 2020; Liu et al., 2020). By September 2021, more than 200 million people had been infected and almost 3 million had died due to SARS‐CoV‐2 infection (World Health Organizatoin (WHO), 2021). The disease, denominated as coronavirus disease 2019 (COVID‐19), is the third recent fatal infection caused by a coronavirus (CoV). Although the origin of this infection is still unknown, all preliminary studies indicate that the origin of the virus is likely zoonotic (Lau et al., 2020; Wong et al., 2020). Before the COVID‐19 pandemic, two other outbreaks produced by CoVs had drawn attention towards viral zoonosis. The first was caused by SARS‐CoV and originated in Guangdong Province, China (Wong et al., 2020), in 2002, while the second was caused by Middle East respiratory syndrome coronavirus and was first detected in Saudi Arabia in 2012. Both viruses originated in bats but had other intermediate hosts (palm civets (Paradoxurus hermaphroditus) (Guan et al., 2003) and dromedary camels (Camelus dromedarius) (Azhar et al., 2014; Reusken et al., 2013), respectively) and subsequently infected humans (Drosten et al., 2003; Zaki et al., 2012). The same process is thought to have been responsible for SARS‐CoV‐2 emergence since some studies have reported a high similarity to a bat coronavirus isolate (Wong et al., 2020; Zhou et al., 2020). However, confirmation of the direct transmission of SARS‐CoV‐2 from bats to humans has not been possible (Wong et al., 2020). What does appear certain is that the virus underwent a period of adaptation in an as yet unidentified animal host, thus facilitating its capacity to jump species boundaries and infect humans (Zhou et al., 2020). Moreover, it is also known that CoVs have a wide distribution in animals, with a high genetic diversity and frequent recombination of their genomes (Ludwig & Zarbock, 2020; Voskarides, 2020; Wang et al., 2020).
These shreds of evidence, together with the fast spread of the virus and its concomitant mortality, have led to considerable concern about the potential capacity of the virus to adapt to other species and become even more transmissible and virulent. Indeed, certain events have confirmed the importance of this concern, such as the detection of new SARS‐CoV‐2 variants associated with farmed mink in Denmark, including 12 cases with a unique variant (‘cluster five variant’), which had a combination of mutations that had not previously been observed (WHO, 2020b). Although the implications of these changes are not yet well understood, preliminary outcomes suggested that the mink‐associated variant identified in these 12 human cases has moderately decreased sensitivity to neutralizing antibodies (Lassaunière et al., 2020).
Furthermore, several cases of natural SARS‐CoV‐2 infection have been identified in animals to date (Delahay et al., 2021). According to the World Organization for Animal Health (OIE), SARS‐CoV‐2 RNA has been sporadically detected in dogs, cats, and ferrets (Ferasin et al., 2021; Gortázar et al., 2021; Hamer et al., 2020; Patterson et al., 2020) from many countries, and also in other animals not considered as pets, such as lions, tigers, and pumas (OIE, 2021). Most of the cases reported have been associated with direct contact between animals and infected humans (Hobbs & Reid, 2020; Ruiz‐Arrondo et al., 2020; Sit et al., 2020). However, natural SARS‐CoV‐2 infection has also been reported in animals with non‐apparent COVID‐19 positive contact (Denis et al., 2020; Hobbs & Reid, 2020; Zhang et al., 2020) or environments in high prevalence settings (Fernández‐de‐Mera et al., 2020) that are accessible to stray animals. Experimental infection trials have also demonstrated that many animals are susceptible to the infection, particularly in the case of cats (Bosco‐Lauth, Hartwig, Porter, Gordy, Nehring, Byas, Woude, et al., 2020; Chen et al., 2020; Shi et al., 2020), hamsters (Sia et al., 2020), and ferrets (Kim et al., 2020; Richard et al., 2020; Shi et al., 2020). Dogs that have undergone experimental inoculation would, however, appear to have a low susceptibility to the virus (Shi et al., 2020), although serological studies suggest that they may be infected under natural conditions (Fritz et al., 2020; Y. Zhao, Yang, et al., 2022).
It is, therefore, essential to study SARS‐CoV‐2 infection in animals during this pandemic since their potential role as reservoirs or transmitters of SARS‐CoV‐2 remains unclear. This is particularly important in the case of pets (and principally cats and dogs), which are in direct contact with their owners and are known to be susceptible to the infection (Abdel‐Moneim & Abdelwhab, 2020; Hossain et al., 2020; Patterson et al., 2020; Shi et al., 2020). In this study, we study the prevalence and seroprevalence of SARS‐CoV‐2 infection in 763 dogs and 753 cats in Spain, which is a high human COVID‐19 prevalence country. In order to reduce the potential for bias, samples were collected from both animals in contact with COVID‐19 positive people and/or compatible symptoms, and random animals for which there was no epidemiological evidence that they had come into contact with the disease.
2. MATERIALS AND METHODS
2.1. Animal sampling and transport
Study enrolment criteria included two kinds of sampling: a selective sampling, which consisted of samples obtained from any pet (cat or dog) living/in contact with confirmed COVID‐19 positive people [household or animal keeper in the case of Animal Protection Centers (APC)] and/or with clinical signs compatible with the disease (i.e. respiratory and digestive symptoms, anorexia, and apathy), and a random sampling (which included randomly selected animals that visited veterinary clinics/hospitals or were located in APCs with unknown COVID‐19 positive contact and health status). Sampling comprised animals from nine Autonomous Communities in Spain, which represent 64.15% of the total population of cats and dogs in the country. The dog and cat census estimate for each Autonomous Community included in this study is shown in Table 1.
TABLE 1.
Dog and cat census estimate for each Autonomous Community used in this study. Source: Veterindustria
| Autonomous Community | Dog census estimate (number of dogs) | Cat census estimate (number of cats) |
|---|---|---|
| Andalucía | 1,621,636 | 754,765 |
| Castilla la Mancha | 394,709 | 76,834 |
| Castilla y León | 447,188 | 340,428 |
| Cataluña | 537,977 | 406,479 |
| Ceuta | 7751 | 4220 |
| Comunidad de Madrid | 832,487 | 491,041 |
| Comunidad Valenciana | 873,343 | 500,658 |
| Navarra | 135,391 | 255,840 |
| País Vasco | 230,621 | 104,203 |
All the samples were obtained from practitioners from clinics or APC using protocols approved by the Complutense University of Madrid's Ethics Committee for Animal Experiments (Project License 14/2020) and were subsequently sent to the VISAVET (Health Surveillance Centre) Center at the Complutense University of Madrid, Madrid, Spain, by a transport company under the regulations stated in the UN3373. Owners/keepers were duly informed regarding the purpose of the study and the data protection policy, and a written consent for each pet was received from their owners/keepers.
Samples included whole blood, sera, nasopharyngeal/oropharyngeal/nasal, and rectal swabs and surface sponges for the detection of environmental viral RNA. Whole blood was collected in ethylenediaminetetraacetic acid tubes, while serum samples were collected in a tube without any anti‐coagulant. Swabs were collected in DeltaSwab Virus 3 ml contained in viral transport media (VTM) (Deltalab S.L., Cataluña, Spain). Dry Sponges 3 M (3 M, Minnesota, USA) were used to collect environmental RNA from the surfaces of the animals (skin and hair). These sponges were pre‐hydrated with 15 ml of an isotonic surfactant liquid that inactivates the virus but preserves the genetic material (Martínez‐Guijosa et al., 2020).
Epidemiological and clinical information regarding each animal was recorded when possible, including information concerning positive COVID‐19 contacts, clinical signs, comorbidities, age, breed, and sex. However, historical information about stray animals was not available in the majority of the cases.
2.2. Virus and cells
SARS‐CoV‐2 MAD6 isolated from a 69‐year‐old male patient in Madrid (Spain) was kindly provided by Dr. Luis Enjuanes from the National Biotechnology Centre at the Higher Council for Scientific Research.
Vero E6 cells (provided by the Carlos III Healthcare Institute, Madrid, Spain or ATCC, Manassas, Virginia) were prepared in order to reproduce the SARS‐CoV‐2 stocks. Cells were incubated at 37°C under 5% CO2 in Gibco Roswell Park Memorial Institute (RPMI) 1640 medium with l‐glutamine (Lonza Group Ltd., Basel, Switzerland) and supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (FBS) (Merck KGaA, Darmstadt, Germany). SARS‐CoV‐2 titres were determined via a tissue culture infectious dose (TCID50) assay.
2.3. RNA extraction and reverse transcription quantitative PCR
SARS‐CoV‐2‐specific RNA was detected using a reverse transcription quantitative PCR (RT‐qPCR) assay. Briefly, nasopharyngeal/oropharyngeal/nasal and rectal swabs contained in VTM were extracted under biosafety level 3 conditions at the VISAVET centre at the University Complutense of Madrid, Spain, using the KingFisher Flex System automated extraction instrument (ThermoFisher, Waltham, MA, USA), using the MagMAX Viral/Pathogen Nucleic Acid Isolation Kit (ThermoFisher) according to the manufacturer's instructions.
The detection of SARS‐CoV‐2 RNA was performed using the envelope protein (E)‐encoding gene (Sarbeco) and two targets (IP2 and IP4) of the RNA‐dependent RNA polymerase gene (RdRp) in an RT‐qPCR protocol established by the World Health Organization according to the guidelines that can be found at https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/technical‐guidance/laboratory‐guidance (Corman et al., 2020). Real‐time RT‐PCR was carried out using the SuperScript III Platinum One‐Step RT‐qPCR Kit (ThermoFisher) according to the protocol described above in a CFX Connect™ Real‐Time PCR Detection System (BioRad, Berkeley, USA). A positive cycle threshold (Ct) cut‐off of 40 cycles was used. A result was considered positive when the sample attained a positive result for at least two of the three targets analyzed and was confirmed by the sequencing of the positive PCR product, according to the OIE guidelines (OIE, 2021).
2.4. Adaptation and validation of a screening enzyme‐linked immunosorbent assay
To select an enzyme‐linked immunosorbent assay (ELISA) for screening of sera, three commercial ELISA tests were evaluated. First, we tried two assays based on the nucleocapsid (N) protein of SARS‐CoV‐2: INgezim COVID 19 DR (Ingenasa, Madrid, Spain) and ID Screen SARS‐CoV‐2 Double Antigen Multi‐species (ID‐Vet, Grabels, France); both tests were performed according to manufacturer´s instructions. For the validation of the assays, 78 sera (45 cats and 33 dogs) from years previous to the pandemic were tested with both tests. A great number of false positives were obtained with both tests (Supporting Information 1). Therefore, we discarded the N protein as a good target for antibody detection in cats and dogs. Cross‐reaction with feline Coronavirus and canine Coronavirus was studied by evaluating all the positive sera with a coronavirus specific‐species ELISA test: INgezim corona feline for cats (Ingenasa) and INgezim corona canine for dogs (Ingenasa). As several false positive sera were negative for the specific coronavirus ELISA, we discarded cross‐reaction with the N protein of both coronaviruses.
Then, an ELISA test based on the receptor‐binding domain (RBD) of the virus was performed, using an indirect ELISA with the SARS‐CoV‐2 RBD protein (Raybiotech, Georgia, USA). The ELISA was adapted to each species by using a specific anti‐species conjugate. Briefly, coated plates were covered with 100 μl of diluted sera (1/40) in PBS containing 0.05% Tween 20 (PBS‐T) and incubated at 37°C for 30 min. The plates were then washed four times, the specific anti‐species HRP‐conjugated IgG (Jackson Immuno Research Laboratories, Cambridgeshire, UK) diluted 1/18,000 in PBS‐T was added, and the solution was incubated at 37°C for 15 min. After four more washes, 100 μl of SureBlue Reserve TMB Microwell Peroxidase Substrate (TMB) (KPL, Gaithersburg, MD, USA) was added, and the plates were incubated in the dark for 10 min. The reaction was stopped by adding 100 μl of 3 M H2SO4 to each well. Absorbance at 450 nm was determined using an Anthos 2001 plate reader (Labtec, Salzburg, Austria). The endpoint cut‐off was determined by the analysis of a receiver operating characteristic curve based on positive divided by negative (P/N) values.
Validation of the adapted ELISA was performed by comparing the results of a representing subsample of sera with the results obtained from the previously validated surrogate neutralization assays for SARS CoV‐2 (GenScript, Inc., NJ, USA) (Perera et al., 2021), which was executed according to the manufacturer's instructions. The subsample consisted of 100 sera, which were evaluated by both assays. Cohen's kappa coefficient (κ) was used to calculate the degree of agreement of both tests using SPSS 22 (IBM, Chicago, USA). Sensitivity and specificity for the adapted RBD ELISA test were calculated against the standard surrogate neutralization assays for SARS CoV‐2. Sensitivity was calculated as the proportion of test‐positive samples over the total number of samples from seropositive animals. Specificity was calculated as the proportion of test‐negative samples over the number of seronegative animals. Positive predictive value (PPV; percentage of animals with a positive test which actually have antibodies), and negative predictive value (NPV; percentage of animals with a negative test which do not have antibodies) were calculated according to Parikh et al. (2008).
All doubtful or positive serum samples were evaluated by employing the virus neutralization test (VNT) to confirm the existence of neutralizing antibodies.
2.5. Virus neutralization test
Sera were tested for neutralizing antibodies against SARS‐CoV‐2 by means of a VNT. Briefly, the VNT was performed in duplicate in 96‐well plates by incubating 25 μl of twofold serially diluted sera with 25 μl of 100 TCID50/ml of SARS‐CoV‐2. The virus‐serum mixture was incubated at 37°C with 5% CO2. At 1‐h post‐incubation, 200 μl of Vero E6 cell suspension was added to the virus‐serum mixtures, and the plates were incubated at 37°C with 5% CO2. The neutralization titres were determined at 3, 5, and 7 days post‐infection. The titre of a sample was recorded as the reciprocal of the highest serum dilution that provided at least 100% neutralization of the reference virus, as determined by the visualization of cytopathic effect (CPE). Cell viability after the VNT was additionally determined by using a violet crystal assay to confirm the results observed by microscopy. At the end of the VNT (7 days post‐infection), the cells were dried, 200 μl of 0.5% crystal violet solution (Sigma‐Aldrich, Missouri, USA) was added, and the solution was incubated at room temperature for 20 min. Finally, the crystal violet solution was removed for the visualization of CPE or cellular tapestry. Cell viability was determined by comparing each well with both the virus and the cell control wells.
2.6. Viral isolation
Specimens that tested positive for qRT‐PCR were subjected to virus isolation in Vero E6 cells. These cells were cultured in RPMI supplemented with 10% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin (growth medium). The cells were then seeded in 96‐well culture plates and cultured at 37°C with 5% CO2 for 2448 h, after which they were inoculated with 10 μl of the direct sample contained in VTM (oronasal or rectal swabs). Mock‐inoculated cells were used as negative controls. The cultured cells were maintained at 37°C with 5% CO2, with a daily observation of CPE and cellular death. After 6 days, the cell cultures were frozen, thawed, and subjected to three passages with inoculations of fresh Vero E6 cell cultures with the lysates, as described above. SARS‐CoV‐2 molecular detection was performed by employing RT‐qPCR on the supernatants from every passage in order to confirm the presence/absence of the virus in the cell culture and virus recovery by means of the decrease in the Ct.
2.7. Whole‐genome sequencing and phylogenetic analysis
Whole‐genome sequencing was performed on the samples with Ct values under 30 or the isolates from those samples in which viral isolation was possible. In the remainder of positive samples, attempts to sequence the spike protein genome were made as described in Barroso‐Arévalo et al. (2021).
Whole‐genome sequences from five positive samples from cats and dogs were obtained by RT‐PCR with 38 primers sets following the protocol described by Paden et al. (2020). Sequence analysis was performed using the Sequencing Analysis software v.5.3.1 (Applied Biosystems), while SeqScape v.2.5 software (Applied Biosystems) was used for sequence assembly using the SARS‐CoV‐2 isolate Wuhan‐Hu‐1, complete genome (GenBank accession number: NC_045512) as a reference genome.
Phylogenetic analysis was performed using MEGA X software (Tamura, 1992). A total of 54 representative sequences were used for the analysis, including sequences from cats and dogs, the reference genome from Wuhan, as well as variants of concern such as the B.1.1.7 variant from the United Kingdom, P.1 variant from Brazil, variant B.1.351 from South Africa, and variant B.1.617.2 from India. The final alignment included 59 sequences and was considered adequate because it was associated with an average amino acid p‐distance (1‐amino acid identity) of 0.014. This value is within the acceptance threshold of <0.8 (Tamura, 1992). From this alignment, the phylogenetic tree was constructed using the maximum likelihood method and Subtree‐Pruning‐Regrafting algorithm and bootstrap testing of 2000 replicates.
Mutations were determined using the CoVsurver mutations app available on the GISAID website (https://www.gisaid.org/). We gratefully acknowledge the various laboratories and contributors of GISAID for providing these SARS‐CoV‐2 sequences.
3. RESULTS
3.1. Demographic and sampling data
A total of 763 dogs and 753 cats were sampled (total n = 1516 animals), representing 3670 samples. Table 2 shows the distribution of the animals based on the type of sample (selective or random) in each Autonomous Community. Figure 1 shows the percentage of the total number of animals (dogs and cats) in each Autonomous Community.
TABLE 2.
Number of animals sampled depending on the type of sampling (selective or random), and the represented percentage in each Autonomous Community
| Autonomous Community | Dogs | Cats | ||||
|---|---|---|---|---|---|---|
| Selective sampling | Random sampling | Total dogs (%) | Selective sampling | Random sampling | Total cats (%) | |
| Andalucía | 105 | 337 | 57.92 | 40 | 293 | 44.22 |
| Castilla la Mancha | 18 | 1 | 2.49 | 20 | 165 | 24.56 |
| Castilla y León | 24 | 4 | 3.66 | 11 | 29 | 5.31 |
| Cataluña | 9 | 25 | 4.45 | 15 | 22 | 4.91 |
| Ceuta | 18 | 0 | 2.35 | 5 | 0 | 0.66 |
| Comunidad de Madrid | 118 | 88 | 26.99 | 63 | 45 | 14.34 |
| Comunidad Valenciana | 0 | 0 | 0 | 16 | 15 | 4.11 |
| Navarra | 3 | 0 | 0.39 | 0 | 0 | 0 |
| País Vasco | 13 | 0 | 1.70 | 14 | 0 | 1.85 |
| Total | 308 | 455 | 100 | 184 | 569 | 100 |
| Total n | 763 | 753 | ||||
| 1516 | ||||||
FIGURE 1.
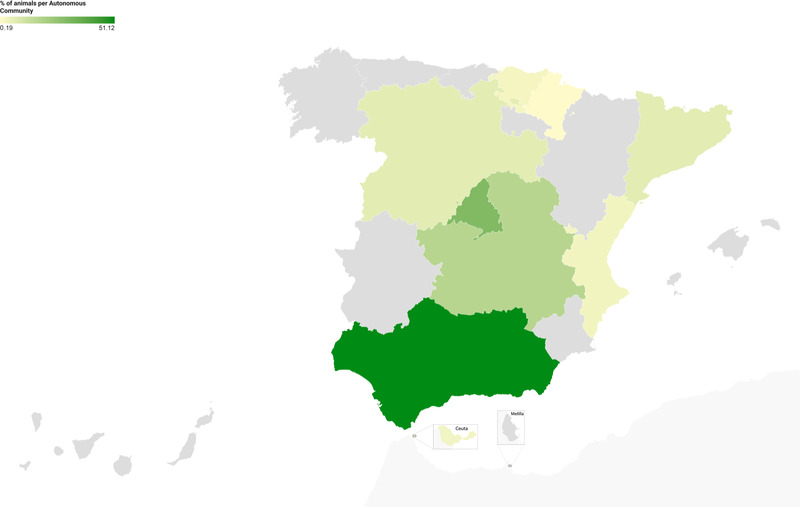
Distribution of percentage of the total number of animals (dogs and cats) in each Autonomous Community in the period elapsed between July 2020 and April 2021
In the selective sampling, 85.16% of the animals had a COVID‐19 positive contact within which 27.50% had COVID‐19 compatible symptoms, while 14.84% displayed COVID‐19 compatible symptoms but did not have COVID‐19 positive known contact. Diverse breeds of dogs and cats were represented; the age of the dogs ranged from 2 months to 18 years, while that of cats ranged from 2 months to 19 years. The clinical signs observed in animals from the selective sampling were respiratory alterations (coughs, sneezing, dyspnoea, lung crackles, nasal discharge, pneumonia, and exercise intolerance), digestive disorders (anorexia, diarrhoea, vomiting, and dysphagia), and non‐specific symptoms (bad coat, dirty hair, and parasitism).
3.2. Infection prevalence and viral isolation
SARS‐CoV‐2 RNA was detected by means of RT‐qPCR and sequencing in eight dogs and four cats. Table 3 shows the PCR positive animals and their distribution depending on the type of sampling, as well as the percentage that these animals represent in each sampling.
TABLE 3.
Positive animals and their distribution depending on the type of sampling, and the percentage represented in each sampling
| Species | PCR positive animals | PCR positive animals in total selective dogs/cats | Positives in total selective dogs/cats (%) | PCR positive animals in total random dogs/cats | % of positives in total random dogs/cats | Positive animals in total dogs/cats (selective + random) | Positives in total animals (%) |
|---|---|---|---|---|---|---|---|
| Dog | 8 | 8 out of 308 | 2.59 | 0 out of 455 | 0 | 8 out of 763 | 1.04 |
| Cat | 4 | 3 out of 184 | 1.63 | 1 out of 569 | 0.17 | 4 out of 753 | 0.53 |
These 12 animals met the OIE criteria of the animal case definition for SARS‐CoV‐2 with regard to positive respiratory and/or rectal swabs (Tables 4 and 5). Two of the positive dogs also tested positive for RNA detection in the case of samples obtained from their surfaces using environmental sponges (Table 4).
TABLE 4.
Virological characteristics of positive dogs to reverse transcription quantitative PCR (RT‐qPCR)
| Ct values of qRT‐PCR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal ID | Species | Type of sampling and origin | Type of sample | E gene | RdRp IP2 | RdRp IP4 | Monitoring qRT‐PCR | Viral isolation | Viral neutralization titre | COVID‐19 positive contact | Animal symptoms compatible with SARS‐CoV‐2 infection |
| +D‐1 | Dog | Selective/Ceuta | Nasal swab | 27.1 | 26.5 | 27.3 | ND | ND | 1/128 (serum = 2 weeks after positive PCR) | Yes | None |
| Rectal swab | ND | ND | ND | ND | X | ||||||
| +D‐2 | Dog | Selective/Madrid | Nasal swab | ND | ND | ND | ND | X | Negative | Yes | None |
| Rectal swab | 35.21 | ND | 36.01 | ND | ND | ||||||
| Blood | ND | ND | ND | ND | X | ||||||
| +D‐3 | Dog | Selective/Madrid | Nasal swab | 37.76 | ND | ND | ND | ND | 1/64 (serum = 10 days after positive PCR) | Yes | None |
| Rectal swab | 32.14 | 30.73 | 33.21 | ND | Yes | ||||||
| Blood | ND | ND | ND | ND | X | ||||||
| +D‐4 | Dog | Selective/Castilla y León | Nasal swab | ND | 37.45 | 36.89 | ND | ND | Serum non‐available | Yes | None |
| Rectal swab | ND | ND | ND | ND | X | ||||||
| +D‐5 | Dog | Selective/Madrid | Nasal swab | ND | ND | ND | ND | X | Negative | Yes | Cough, lung crackles. Suspected brachycephalic syndrome |
| Rectal swab | 37.50 | ND | 35.80 | ND | ND | ||||||
| Blood | ND | ND | ND | ND | X | ||||||
| Saliva | ND | ND | ND | X | X | ||||||
| Loin skin | 34.92 | 36.55 | 35.10 | X | X | ||||||
| Foot skin | 35.82 | 32.62 | 34.46 | X | X | ||||||
| +D‐6 | Dog | Selective/Madrid | Nasal swab | 37.78 | ND | 34.28 | ND | ND | Negative | Yes | Diarrhoea |
| Rectal swab | ND | ND | ND | ND | X | ||||||
| Blood | ND | ND | ND | ND | X | ||||||
| +D‐7* | Dog | Selective/Madrid | Nasal swab | 20.05 | 19.78 | 19.89 | Positive (average Ct 20.21)* | Yes | 1/256 (serum = 19 days after the first positive PCR) | Yes | None |
| Rectal swab | 26.09 | 25.60 | 26.08 | Positive (average Ct 33.55)* | Yes | ||||||
| Blood | ND | ND | ND | ND | X | ||||||
| Hair and skin | 33.40 | 34.49 | 33.04 | Positive (average Ct 25.56)* | X | ||||||
| +D‐8 | Dog | Selective/Andalucía | Nasal swab | ND | 32.11 | 33.12 | Non‐available | ND | 1/128 (serum = same day as positive PCR) | Yes | None |
| Rectal swab | ND | ND | ND | Non‐available | X | ||||||
| Blood | ND | ND | ND | Non‐available | X | ||||||
Abbreviation: ND, not detected; SARS‐CoV‐2, severe acute respiratory syndrome‐related coronavirus 2.
Monitoring PCR was performed 2 days after the original PCR.
TABLE 5.
Virological characteristics of positive cats to reverse transcription quantitative PCR (RT‐qPCR)
| Ct values of qRT‐PCR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal ID | Species | Type of sampling and origin | Type of sample | E gene | RdRp IP2 | RdRp IP4 | Monitoring qRT‐PCR | Viral isolation | Viral neutralization titre | COVID‐19 positive contact | Animal symptoms |
| +C‐1 | Cat | Random/Madrid | Oropharyngeal swab | 32.17 | 30.14 | 33.03 | Positive (average Ct 32.78) | Yes* | Negative | No | History of diarrhoea secondary to chronic intestinal inflammation |
| Rectal swab | 34.84 | ND | 33.58 | ND | ND | ||||||
| Blood | ND | ND | ND | ND | X | ||||||
| +C‐2 | Cat | Selective/Castilla y León | Oropharyngeal swab | 31.99 | 30.78 | 30.18 | ND | Yes | 1/32 (serum = same day as positive PCR) | Yes | None |
| Rectal swab | ND | ND | ND | ND | X | ||||||
| Blood | ND | ND | ND | ND | X | ||||||
| +C‐3 | Cat | Selective/Castilla y León | Nasal swab | ND | 36.89 | 37.99 | ND | ND | Serum non‐available | Yes | None |
| Rectal swab | ND | ND | ND | ND | X | ||||||
| +C‐4 | Cat | Selective/Madrid | Oropharyngeal swab | 30.2 | ND | 30.8 | ND | ND |
1/64 (serum = same day as positive PCR) 1/32 (serum = 15 days after positive PCR) |
Yes | Leukaemia and feline immunodeficiency viruses positive Nasal discharge, anorexia, hypersomnia. Enlarged retropharyngeal lymph node |
Abbreviations: Ct, cycle threshold; ND, not detected.
Virus isolation was possible from the first swab collected.
All the animals that tested positive for RT‐qPCR were re‐tested as soon as possible after the positive result had been obtained. One dog (D+‐7), which resulted positive with a high viral load based on PCR results, was positive again when it was resampled 3 days after the original sampling. This dog was negative when re‐tested 19 days after the initial sampling. One cat (C+‐1) attained a positive result for the nasopharyngeal swab collected 14 days after the initial PCR (Table 5).
Attempts were made to isolate the virus from all the RT‐qPCR positive swabs. SARS‐CoV‐2 was isolated from four animals: from the rectal swab of one dog (+D‐3), for which CPE was observed in the second and third passages, and virus recovery was confirmed by means of PCR (Ct value reduction from cell suspension of first passage to second and third passages); from the nasopharyngeal swab of one cat (+C‐1), for which CPE was observed in the second and third passages and virus recovery was confirmed by means of PCR; from the nasopharyngeal swab of another cat (+C‐2), for which no CPE was noted on day 7 but virus recovery was confirmed by means of PCR; and from the nasal and rectal swab of another dog (+D‐7), with CPE noted in all passages, in which virus recovery was confirmed by means of PCR. Detailed description about samples in which viral isolation was possible is presented in Supporting Information 1 and 2.
3.3. Validation of the adapted RBD ELISA
Validation of adapted RBD ELISA was performed using a subsample of 100 sera (Table 6). Before evaluation with the adapted RBD ELISA, these sera were also analyzed using the previously validated surrogate neutralization assay for SARS CoV‐2 described above. Based on this comparative assay, sensitivity and specificity were 93% and 95%, respectively. Both tests had a kappa agreement of 0.827.
TABLE 6.
Two‐by‐two table used to determine the sensitivity and specificity of the adapted receptor‐binding domain (RBD) enzyme‐linked immunosorbent assay (ELISA) test comparing to the validated surrogate neutralization assay
| Serum result | Surrogate neutralization assay test positive | Surrogate neutralization assay test negative | Total test results |
|---|---|---|---|
| RBD ELISA test positive | 15 (TP) | 4 (FP) | 19 (total test positive) |
| RBD ELISA test negative | 1 (FN) | 80 (TN) | 81 (total test negative) |
| Total samples analyzed | 16 | 84 | 100 (total subsample) |
Abbreviations: FN, false negatives; FP, false positives; TN, true negatives; TP, true positives.
3.4. Antibody detection by employing the RBD ELISA and VNT
A total of 1488 sera were evaluated using the screening RBD ELISA. Of these sera, 59 showed a positive or doubtful result and were subsequently evaluated by VNT.
A total of 20 dogs (Table 7), 16 of these from the selective sampling and four from the random sampling, had neutralizing antibodies, with titres ranging from 1/16 to 1/128. Four of these dogs were also positive for RT‐qPCR (+D‐1, +D‐3, +D‐7, and +D‐8, Tables 4 and 7) and had neutralizing antibodies 14, 10, and 19 days after testing positive for PCR and the same day as the positive PCR, respectively. Two of the other VNT positive dogs from the selective sampling (VNT D‐1 and VNT D‐2) were living with the PCR‐positive dogs mentioned above (+D‐1 and +D‐3, respectively). Other 10 VNT‐positive dogs had also been in contact with COVID‐19 positive people, and only one of these had respiratory signs (cough and dyspnea). The other four VNT‐positive dogs belonged to the random sampling, so they did not have any apparent COVID‐19 positive contact nor compatible symptoms.
TABLE 7.
Positive animals for virus neutralization test (VNT)
| Animal ID | Species | VNT titre | PCR result | Positive contact | Symptoms |
|---|---|---|---|---|---|
| +D‐1 | Dog | 1/128 | Positive | Yes | No |
| +D‐3 | Dog | 1/64 | Positive | Yes | No |
| +D‐7 | Dog | 1/256 | Positive | Yes | No |
| +D‐8 | Dog | 1/128 | Positive | Yes | No |
| VTN‐D‐1 | Dog | 1/32 | Negative | Yes | No |
| VTN‐D‐2 | Dog | 1/64 | Negative | Yes | No |
| VTN‐D‐3 | Dog | 1/32 | Negative | Yes | No |
| VTN‐D‐4 | Dog | 1/64 | Negative | Yes | No |
| VTN‐D‐5 | Dog | 1/16 | Negative | Yes | No |
| VTN‐D‐6 | Dog | 1/128 | Negative | Yes | No |
| VTN‐D‐7 | Dog | 1/64 | Negative | Yes | No |
| VTN‐D‐8 | Dog | 1/128 | Negative | Yes | No |
| VTN‐D‐9 | Dog | 1/64 | Negative | Yes | No |
| VTN‐D‐10 | Dog | 1/128 | Negative | Yes | No |
| VTN‐D‐11 | Dog | 1/32 | Negative | Yes | Yes |
| VTN‐D‐12 | Dog | 1/64 | Negative | Yes | No |
| VTN‐D‐13 | Dog | 1/32 | Swab non‐available | No | No |
| VTN‐D‐14 | Dog | 1/64 | Negative | No | No |
| VTN‐D‐15 | Dog | 1/32 | Swab non‐available | No | No |
| VTN‐D‐16 | Dog | 1/128 | Swab non‐available | No | No |
| +C‐2 | Cat | 1/32 | Positive | Yes | No |
| +C‐4* | Cat | 1/64 and 1/32* | Positive | Yes | Yes |
| VTN‐C‐1 | Cat | 1/256 | Negative | Yes | Yes |
| VTN‐C‐2 | Cat | 1/128 | Negative | Yes | No |
| VTN‐C‐3 | Cat | 1/32 | Negative | Yes | Yes |
| VTN‐C‐4 | Cat | 1/64 | Negative | Yes | No |
| VTN‐C‐5 | Cat | 1/32 | Negative | Yes | Yes |
| VTN‐C‐6 | Cat | 1/16 | Negative | Yes | No |
| VTN‐C‐7 | Cat | 1/128 | Swab non‐available | Yes | No |
| VTN‐C‐8 | Cat | 1/64 | Negative | Yes | No |
| VTN‐C‐9 | Cat | 1/32 | Negative | No | Yes |
| VTN‐C‐10 | Cat | 1/64 | Negative | No | Yes |
| VTN‐C‐11 | Cat | 1/64 | Negative | Yes | No |
| VTN‐C‐12 | Cat | 1/128 | Negative | No | No |
Sera from animal +C‐4 was available at two points: when the original PCR positive sample was taken and 15 days after the original sampling.
On the other hand, 14 cats had neutralizing antibodies (Table 7). One of these cats was positive for RT‐qPCR and had neutralizing antibodies in the serum collected at the same time as the positive PCR (+C‐2) (titter 1/32) (Table 3). Another PCR‐positive cat (+C‐4) also had neutralizing antibodies, with a titre of 1/64 obtained on the same day as the positive PCR and 1/32 15 days after the positive PCR. Other 11 VNT‐positive cats (which were negative for PCR) were included in the selective sampling as they lived with a COVID‐19 positive owner and/or had compatible symptoms (Table 6). Only one VNT‐positive cat belonged to the random sampling (VNT C‐12) and was a stray cat.
3.5. Detection of environmental viral RNA
We employed special sponges with a surfactant liquid that allows for viral inactivation in order to assess the presence of environmental viral RNA in animal surfaces. In addition to +D‐5 and +D‐7 (Table 4), five animals from the selective sampling tested positive with regard to RNA detection of SARS‐CoV‐2 on surface samples collected using environmental sponges. The first was a cat that lived with positive COVID‐19 owners and tested positive with regard to RNA detection on its hair surface but negative with regard to nasal and rectal swabs. The second was a dog whose owner was positive for COVID‐19 disease; the nasal and rectal swabs and blood of this dog tested negative for SARS‐CoV‐2 RNA detection by RT‐qPCR. The third was a cat with a history of severe hypersensitivity of unknown origin living with COVID‐19 positive owners. Despite the positive result obtained using the environmental sponge, the cat tested negative when employing nasal and rectal swabs. The fourth and the fifth were two dogs living with COVID‐19 positive owners, although nasal and rectal swabs, along with the blood from these dogs, tested negative in both cases. However, one of these dogs was positive for VNT (VTN‐D‐8, see Table 7). All the environmental samples showed Ct values above 30 except for the second sample taken from the hair and skin of +D‐7 (average Ct: 25.56).
3.6. Sequencing analysis
Five complete genome sequences were obtained from the nasal swab of +D‐1, the rectal swab of +D‐3 (viral culture isolate), the nasal swab of +D‐7, and the oropharyngeal swabs of +C‐1 and +C‐2 (viral culture isolates). GenBank accession numbers are shown in Table 8.
TABLE 8.
Whole‐genome sequences obtained from three positive dogs and two positive cats, corresponding GenBank accession numbers and list of mutations found in the genome according to CoVsurver mutation app available at GISAID
| Animal ID | GenBank accession number | List of mutations displayed in structure (nearest residue if in loop/termini region) in the genome |
|---|---|---|
| +D‐1 | MZ902039 | D614G, Q675del(674), T676del(674), 677del(674), T678del(674), N679del(674) |
| +D‐3 | MZ902042 | D215A, T573I, D614G, Q675del(674), T676del(674), Q677del(674), T678del(674), N679del(674) |
| +D‐7 | MZ914594 | H69del, V70del, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H, Y144del, D138H, E619K |
| +C‐1 | MZ901915 | D614G |
| +C‐2 | MZ902033 | T573I D614G, Q675del(674), T676del(674), Q677del(674), T678del(674), N679del(674) |
Evolutionary relationships among the whole genomes were inferred using maximum likelihood based on the general time‐reversible model (Tamura, 1992). The analysis involved 59 nucleotide sequences, including first, second, third, and non‐coding codon positions. All positions with less than 95% site coverage were eliminated. That is, fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position. In the end, 29,514 positions were analyzed. The bootstrap consensus tree inferred from 2000 replicates (Kumar et al., 2018) was taken to represent the evolutionary history of the taxa analyzed. Branches were collapsed if the corresponding partitions occurred in fewer than 50% of bootstrap replicates. Initial tree(s) for the heuristic search were obtained automatically by applying neighbour‐joining and BioNJ algorithms to a matrix of pairwise distances estimated using maximum composite likelihood and then selecting the topology with a better log‐likelihood value. Differences in the rate of evolution among different sites were modelled using a discrete gamma distribution (two categories, +G parameter = 0.059).
Phylogenetic analysis showed that sequences from +C‐1 and +C‐2 were very similar and clustered with the genome from +D‐3. Sequence from +D‐1 was also close to the sequences cited above (Figure 2), and the four sequences were also close to the reference genome from Wuhan. On the other hand, sequence from +D‐7 was identified as the variant of concern B.1.1.7, clustering with sequences of this variant (Figure 2). Detailed results about this case are reported in Barroso‐Arévalo et al. (2021).
FIGURE 2.

Maximum likelihood phylogeny was estimated with n = 59 complete genomes sequences from the current (2019−2021) SARS‐CoV‐2 retrieved from GISAID. Open square indicates references from this study; open circle indicates the reference genome from Wuhan. Number along branch represents bootstrap score. Scale bar represents expected substitutions per nucleotide site
Mutation analysis on the CoVsurver mutations app detected several mutations in the sequences obtained (Table 8).
4. DISCUSSION
This is, to our knowledge, the first large‐scale study on serological, epidemiological, and virological identification, including viral isolation of SARS‐CoV‐2 in cats and dogs from different regions of Spain. Our results confirm that pets (dogs and cats) can be infected by SARS‐CoV‐2 and are even able to develop neutralizing antibodies against the virus, as other authors have found (Dias et al., 2021; Hamer et al., 2020; Patterson et al., 2020; Ruiz‐Arrondo et al., 2020). Here, we have also isolated the virus from dog and cat samples for the first time in Spain, demonstrating that infectious virus can be isolated despite high Cts. This indicates that the virus can successfully replicate in these animals in natural conditions even though not producing any clinical symptoms. Nowadays, whether or not the implications of these facts are critical for the development of the pandemic is still under examination, but the surveillance of COVID‐19 status in animals is necessary given the zoonotic origin of the disease and the current epidemic situation. The WHO has recently supported the hypothesis that the virus originated in a natural reservoir (probably bats), but then jumped to humans from another small mammal (WHO, 2020a). Since the intermediary host has not yet been identified, pets cannot be dismissed as potential reservoirs, keeping in mind the results reported by this study and others (Abdel‐Moneim & Abdelwhab, 2020; Hobbs & Reid, 2020; Ruiz‐Arrondo et al., 2020; Y. Zhao, Yang, et al., 2022).
At present, there is no evidence of SARS‐CoV‐2 transmission from pets to humans, although this has been confirmed in the case of minks (Hammer et al., 2021), with a large number of outbreaks reported (OIE, 2021), showing that mink farms could represent a serious, unrecognized animal reservoir for SARS‐CoV‐2 (Hammer et al., 2021). Mink‐to‐human transmission has also been demonstrated (Oude Munnink et al., 2021) and was associated with the development of a new mink‐related variant (WHO, 2020b). On the contrary, none of these phenomena have as yet been described in pets. However, transmission between cats via respiratory droplets has been demonstrated (Halfmann et al., 2020; Shi et al., 2020), although the serial passaging of the virus between cats naturally attenuated the viral transmissibility, as has been reported by Bao et al. (2021). In the case of dogs, data from experimental studies suggest that they can also become infected, but might not spread the virus to other dogs as easily as occurs with cats (Hobbs & Reid, 2020; Shi et al., 2020; Sit et al., 2020). However, and with the knowledge we have attained to date, the possibility of pet‐to‐pet and pet‐to‐human spread cannot be completely ruled out. Great caution is, thus, necessary with regard to this topic, and the scientific community must continue to study this potential transmission route.
We found a low prevalence of infected pets on the basis of RNA viral detection, which coincides with the results obtained in other studies (Hamer et al., 2020; Patterson et al., 2020; Ruiz‐Arrondo et al., 2020). However, the possibility that a larger number of animals could have been infected cannot be discarded despite the negative results obtained using PCR. The features of our sampling signified that there was a critical delay between the COVID‐19 positive contact and the moment at which the veterinarian was able to perform the sampling. Restriction measures and quarantine force owners to stay at home, and they cannot, therefore, go to the veterinary hospital/clinic to get their pet sampled. It is thus possible that more animals were infected, but as the period in which cats and dogs shed SARS‐CoV‐2 may be very short, along with the fact that they may have undergone asymptomatic infection, we were unable to pinpoint the moment of acute infection. One proof of this is that we detected neutralizing antibodies in several animals that tested negative when employing PCR (16 out of 20 VNT‐positive dogs were negative for PCR, and 12 out of 14 VNT‐positive cats were negative for PCR), which shows that these animals had an active infection at some point. In future work, it would be advisable to perform a more active sampling, as has occurred in other studies, in order to find active virus infection (Hamer et al., 2020; Temmam et al., 2020), as well as to perform experimental infection assays. The low viral loads found in this study may also be explained by the delay between the positive contact and the pet sampling. For instance, +D‐7 is a case that illustrates this fact perfectly. The owners of this dog were suffering an acute SARS‐CoV‐2 infection at when the dog was sampled. In this case, the veterinarian took the samples at its house while the family was under quarantine. This allows us to detect higher viral loads in the animal (nasal swab average Ct: 19.90). In this case, we were also able to isolate the virus from the nasal and rectal swab of this dog, demonstrating that the dog was suffering an active viral infection at the time of sampling. As reported in Barroso‐Arévalo et al. (2021), this dog was infected with the B.1.1.7 variant of SARS‐CoV‐2, which may explain the high loads detected in the samples taken from this dog. This case coincided with a moment in which B.1.1.7 variant was very prevalent in the country, facilitating the transmission between infected humans and their pets. Further discussion about this case can be found in Barroso‐Arévalo et al. (2021). Although we did not detect any other variant of concern in positive animals, we cannot discard that these variants have higher rates of transmission between humans and animals. The rest of the sequences obtained from positive animals (two dogs and two cats) were identified as common SARS‐CoV‐2 strains, not showing any significant mutation. These results remark the importance of monitoring the presence of variants in sequences from animals since the origin of the virus was probably a spillover from some animals not yet identified (WHO, 2020a). In addition, a mink‐related variant was reported in an outbreak in Denmark (WHO, 2020b).
As expected, most of the positive cases to PCR were included in the selective sampling and had contact with COVID‐19 positive people (11/12). The same occurred in the case of animals that tested positive for neutralizing antibody detection. In fact, two of the VNT‐positive dogs were living with two other animals (also dogs) that tested positive for PCR, respectively. In these particular cases, we were unable to elucidate whether a pet‐to‐pet transmission had occurred or whether the positive human contacts were the source of the infection for these animals. According to the experimental studies carried out to date, human‐to‐pet transmission would appear to be the most likely pathway, since dog‐to‐dog transmission has not yet been demonstrated (Hobbs & Reid, 2020). The fact that more dogs were positive for both PCR and VNT despite their lower susceptibility may be explained by the species behaviour. Dogs usually come into closer contact with their owners than cats, thus facilitating viral transmission through kisses, licks, and other kinds of contact.
Interestingly, one cat (C+−1) belonging to the random sampling tested positive for PCR with regard to both oropharyngeal and rectal swabs. We were able to isolate the virus from the oropharyngeal sample obtained from this animal, which demonstrated that an active infection was occurring. Apparently, the owners of this cat did not have any symptoms compatible with COVID‐19 disease and had not been subjected to a PCR test, but we suspect that they may have been asymptomatic. These results highlight that animals can get infected even in supposedly virus‐free environments. In this context, it is possible to assume that the cat could have been exposed to the virus at any time. Although the cat had a history of digestive problems (diarrhoea secondary to chronic intestinal inflammation), these symptoms did not appear to have been triggered by SARS‐CoV‐2 infection since they were previous to the sampling time. Besides, it was possible to isolate the virus from its oropharyngeal swab, which indicated that the cat was suffering from an active infection. Another fact that supports this hypothesis is that the oropharyngeal swab was again positive when the cat was re‐tested 14 days after the initial positive PCR, suggesting a persistent but asymptomatic infection. This cat, however, did not have any neutralizing or non‐neutralizing antibodies when its sera were tested using VNT and the RBD ELISA, respectively.
It was also possible to isolate the virus from the nasopharyngeal swab of another cat (C+−2), from the rectal swab of one dog (D+−3), and from the nasal and rectal swabs from another dog (D+−7). The three animals had neutralizing antibodies with a titre of 1/32, 1/64, and 1/256, respectively. Despite the high Cts in the original PCR, we were able to isolate the virus, which demonstrates that the virus can be infectious even when the PCR indicates a low viral load. This is, to our knowledge, the first time that virus isolation is performed from animal samples under natural conditions in Spain.
With regard to symptomatology in pets, our results support the belief that most of the SARS‐CoV‐2 virus infections in cats and dogs do not trigger any symptoms, with the exception of immunocompromised animals. Although three PCR‐positive animals had digestive and/or respiratory clinical signs (+D‐5, +C‐1, and +C‐4), the symptoms appear to be explained by comorbidities in most cases. Only in one cat (+C‐4) that was immunosuppressed (the cat was positive for leukaemia and feline immunodeficiency viruses) it is possible that clinical signs were triggered by SARS‐CoV‐2 infection. This cat had a nasal discharge, anorexia, hypersomnia, and an enlarged retropharyngeal lymph node just 5 days after its owner was shown to be COVID‐19 positive. Unfortunately, the animal could not be sampled until 15 days after the initial symptoms appeared, which could explain the high Ct found in the PCR (30.2). The cat had likely already been eliminating the virus at the time of sampling. Fifteen days after the first positive PCR, the animal was resampled, and a negative result was obtained. Serum from the day of the positive PCR showed neutralizing antibodies (titre: 1/64), with a light but not significant decrease in the antibody titre when the cat was resampled (titre: 1/32). This evidences that the cat had suffered from an active SARS‐CoV‐2 infection and had, according to its antibodies, developed an effective immune response. Another interesting case was +D‐7. Both nasal and rectal swabs showed positive results for PCR with high viral loads based on Ct values. However, the animal did not show any clinical signs during the course of the infection. These results are in line with those reported by other authors, where dogs experimentally infected did not display any symptoms (Bosco‐Lauth, Hartwig, Porter, Gordy, Nehring, Byas, Vandewoude, et al., 2020; Shi et al., 2020) despite being susceptible to the virus.
Environmental RNA was detected from the surface of several animals that were living with COVID‐19 positive owners. The methodology used consisted of dry sponges pre‐hydrated with a surfactant liquid that inactivates the virus but allows for RNA preservation. This method has been previously used for Mycobacterium tuberculosis complex detection (Martínez‐Guijosa et al., 2020), African swine fever (data not shown), as well as environmental SARS CoV‐2 RNA (Fernández‐de‐Mera et al., 2020), with successful results. Virus inactivation by the surfactant liquid is a great asset in SARS‐CoV‐2 environmental evaluation since samples can be processed in a BSL2 area as the virus is not infectious anymore. In our study, we have detected SARS‐CoV‐2 RNA in the surface (hair and skin) of seven animals. Two of them (+D‐5 and +D‐7) were also positive for rectal and/or nasal swabs, respectively. As both animals were living with COVID‐19 positive owners, we could not elucidate if the animal shedding was the source of the environmental RNA. The other five cases of environmental detection were detected in animals from the selective sampling, living with COVID‐19 positive owners, but negative for PCR and neutralizing antibody detection, except for one dog, which was positive for VNT (VTN‐D‐8, see Table 7). Therefore, this dog was previously exposed to the virus, was infected, and recovered from the infection, developing neutralizing antibodies. Although the environmental RNA viral loads detected in most of the cases were low (Cts above 30), we cannot discard the role of these animals as potential carriers of the virus on their surfaces. Hence, this methodology may be a good approach for the detection of environmental RNA and exposure to SARS‐CoV‐2 in pets.
On the other hand, it is also important to note that N protein‐based ELISA tests showed specificity problems, as reported before (Zhao, Schuurman et al., 2022). This fact may be a consequence of a cross‐reaction between some other coronaviruses which infect animals without triggering any clinical disease. It is known that several viruses from Coronaviridae family can infect animals (in this case, cats and dogs) (Haake et al., 2020). Notable coronaviruses of companion animals include feline enteric coronavirus (FCoV), feline infectious peritonitis virus, and canine enteric coronavirus (CCoV). It has been previously demonstrated that antigenic cross‐reactivity between SARS‐CoV‐2 and FCoV type N proteins exists (S. Zhao, Schuurman, et al., 2021). Taken into account the endemic nature of FCoV infection in cats, some of the false positive detected in cat sera may be consequence of cross‐reaction with this infection. However, as described above, no cross‐reaction was detected for both N‐based ELISA tests with the specific‐species coronavirus in our case since several false positive sera were negative for FCoV and CCoV ELISA tests in cats and dogs, respectively. Therefore, we suspect that other coronavirus not identified yet may also be involved in the non‐specific outcomes results obtained with the N protein. Based on these outcomes, the N protein does not appear to be a good target for anti‐SARS‐CoV‐2 antibody detection in cats and dogs.
Regarding seroprevalence, we found neutralizing antibodies in a total of 34 animals (20 dogs and 14 cats) using VNT. Five of these animals were included in the random sampling (four dogs and one cat), so they did not have any apparent contact with COVID‐19 positive people. The four dogs were owned pets, while the cat was a stray animal. These outcomes evidence that these animals were exposed to the virus at some time, probably by environmental contamination or asymptomatic owners in the case of pets, suffered from asymptomatic infection, and developed an immune response based on neutralizing antibodies. Seroprevalence in stray animals has been previously reported by others authors in Wuhan, China (Y. Zhao, Yang, et al., 2022); and Rio de Janeiro, Brazil (Dias et al., 2021). All of this demonstrates that human‐to‐animal transmission events are not only restricted to domestic animals and therefore highlight the importance of performing surveillance in this kind of animals.
All these results evidence that cats and dogs in close contact with people who are positive for the virus may develop a SARS‐CoV‐2 infection, but also when they are not apparently exposed to the virus, as our seroprevalence results showed. However, our findings are encouraging, since only a low percentage of the animals sampled attained a positive result for PCR and/or neutralizing antibody detection, indicating that infection in pets would appear to be anecdotic. Nevertheless, it is still advisable to continue monitoring COVID‐19 status in pets, given the zoonotic origin and the particular and not fully known characteristics of the virus. The South Korean Government recently announced that it will test any pet that has been in contact with a COVID‐19 person and/or has had any symptoms that are compatible with the disease. In the case of a positive result, the pet must be quarantined for 14 days. Other countries are performing similar studies on pets. Although there is no regulation in this respect in Spain, more tests should be conducted on pets, especially those living with positive families. Preventive measures should be taken by owners who are COVID‐19 positive (i.e. they should avoid kissing their pets and decrease unnecessary contact). By simply implementing these measures, SARS‐CoV‐2 infected people could decrease the risk of their pets becoming infected.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We want to thank all the pet owners and veterinarians who participated in this study (Cv Ayavet, CV Confivet, CV Mediterraneo, CV Alcorisa, CV Blackcan, CV Altamira, HCV Universidad Complutense de Madrid, CV Vetsia, CV Torrelodones, CV Parla Este, Gattos Centro Clínico Felino, Hospital Guardiamar, HC León, Hospital Felino Madrid, CV Diagnosfera/Cardiosonic, CV Felina Barcelona, CV Delicias, CV Poveda, Boycan CV, CV La Huella, CV Kato clínica felina, CV Guadalcacín, CV Barbate, CV Lidercan, CV Salteras, CV Cuenca Minera, HCV Universidad de Córdoba, CV Coimbra, CV Torrejón de Ardoz, Hospital Veterinari Canis, CV Petconnection, CMV Pio 109, CV Islas, CV La Serna, CV Ayavet, CV Mendibiru, CV del Val, CV Mundo Animal, CV Rosa Luxemburgo, CV San Isidoro, Equican, Hospital Veterinario Ciudad Real and Infantes Veterinarios (Castilla‐La Mancha)), as well as all the Animal Protection Centers that collaborated during the course of the study (CPA Mancomunidades Henares‐Jarama, CPA Madrid Sur, CPA Zoosanitario de Sevilla, CPA Zoosanitario de Ceuta, CPA León, CPA del Ayuntamiento de Pamplona, CPA Animal Domus Misas Costa, SPAP Alicante, CPA de Córdoba, Centro de acogida Fundación Camp de Tarragona, CPA de Huelva, Sociedad Protectora de Animales y Plantas de Gipuzkoa, CPA los Cantiles, CPA de Málaga, Centro Zoosanitario y Bienestar de Córdoba, Centro Zoosanitario Municipal de Almeria). We are also grateful to Dr. Luis Enjuanes from the National Biotechnology Centre (CNB) at the Higher Council for Scientific Research (CSIC) for kindly providing us the virus.
This research was funded by the Institute of Health Carlos III (ISCIII), project “Estudio del potencial impacto del COVID19 en mascotas y linces” (reference: COV20/01385).
Barroso‐Arévalo, S. , Barneto, A. , Ramos, Á. M. , Rivera, B. , Sánchez, R. , Sánchez‐Morales, L. , Pérez‐Sancho, M. , Buendía, A. , Ferreras, E. , Ortiz‐Menéndez, J. C. , Moreno, I. , Serres, C. , Vela, C. , Risalde, M. Á. , Domínguez, L. , & Sánchez‐Vizcaíno, J. M. (2022). Large‐scale study on virological and serological prevalence of SARS‐CoV‐2 in cats and dogs in Spain. Transboundary and Emerging Diseases, 69, e759–e774. 10.1111/tbed.14366
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abdel‐Moneim, A. S. , & Abdelwhab, E. M. (2020). Evidence for SARS‐CoV‐2 infection of animal hosts. Pathogens, 9(7), 529. 10.3390/pathogens9070529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar, E. I. , El‐Kafrawy, S. A. , Farraj, S. A. , Hassan, A. M. , Al‐Saeed, M. S. , Hashem, A. M. , & Madani, T. A. (2014). Evidence for camel‐to‐human transmission of MERS coronavirus. New England Journal of Medicine, 370(26), 2499–2505. 10.1056/NEJMoa1401505 [DOI] [PubMed] [Google Scholar]
- Bao, L. , Song, Z. , Xue, J. , Gao, H. , Liu, J. , Wang, J. , Guo, Q. , Zhao, B. , Qu, Y. , Qi, F. , Gong, S. , Liu, M. , Lv, Q. , Li, D. , Han, Y. , Zhao, W. , Deng, S. , Liu, Y. , Xiang, Z. , … Qin, C. (2021). Susceptibility and attenuated transmissibility of SARS‐CoV‐2 in domestic cats. The Journal of Infectious Diseases, 223, 1313–1321. 10.1093/infdis/jiab104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso‐Arévalo, S. , Rivera, B. , Domínguez, L. , & Sánchez‐Vizcaíno, J. M. (2021). First detection of SARS‐CoV‐2 B.1.1.7 variant of concern in an asymptomatic dog in Spain. Viruses, 13(7), 1379. https://www.mdpi.com/1999‐4915/13/7/1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco‐Lauth, A. M. , Hartwig, A. E. , Porter, S. M. , Gordy, P. W. , Nehring, M. , Byas, A. D. , Vandewoude, S. , Ragan, I. K. , Maison, R. M. , & Bowen, R. A. (2020). Experimental infection of domestic dogs and cats with SARS‐CoV‐2: Pathogenesis, transmission, and response to reexposure in cats. Proceedings of the National Academy of Sciences, 117(42), 26382. 10.1073/pnas.2013102117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco‐Lauth, A. M. , Hartwig, A. E. , Porter, S. M. , Gordy, P. W. , Nehring, M. , Byas, A. D. , Woude, S. V. , Ragan, I. K. , Maison, R. M. , & Bowen, R. A. (2020). Pathogenesis, transmission and response to re‐exposure of SARS‐CoV‐2 in domestic cats. bioRxiv. 10.1101/2020.05.28.120998 [DOI] [PMC free article] [PubMed]
- Chen, D. , Sun, J. , Zhu, J. , Ding, X. , Lan, T. , Zhu, L. , Xiang, R. , Ding, P. , Wang, H. , Wang, X. , Wu, W. , Qiu, J. , Wang, S. , Li, H. , An, F. , Bao, H. , Zhang, L. , Han, L. , Zhu, Y. , & Xu, X. (2020). Single‐cell screening of SARS‐CoV‐2 target cells in pets, livestock, poultry and wildlife. bioRxiv. 10.1101/2020.06.13.149690 [DOI]
- Corman, V. M. , Landt, O. , Kaiser, M. , Molenkamp, R. , Meijer, A. , Chu, D. K. , Bleicker, T. , Brünink, S. , Schneider, J. , Schmidt, M. L. , Mulders, D. G. , Haagmans, B. L. , Van Der Veer, B. , Van Den Brink, S. , Wijsman, L. , Goderski, G. , Romette, J.‐L. , Ellis, J. , Zambon, M. , … Drosten, C. (2020). Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveillance: Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin, 25(3), 2000045. 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahay, R. J. , De La Fuente, J. , Smith, G. C. , Sharun, K. , Snary, E. L. , Flores Girón, L. , Nziza, J. , Fooks, A. R. , Brookes, S. M. , Lean, F. Z. X. , Breed, A. C. , & Gortazar, C. (2021). Assessing the risks of SARS‐CoV‐2 in wildlife. One Health Outlook, 3(1), 7. 10.1186/s42522-021-00039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis, M. , Vandeweerd, V. , Verbeeke, R. , Laudisoit, A. , Reid, T. , Hobbs, E. , Wynants, L. , & Van Der Vliet, D. (2020). COVIPENDIUM: Information available to support the development of medical countermeasures and interventions against COVID‐19. Transdisciplinary Insights. 10.5281/zenodo.4072014 [DOI] [Google Scholar]
- Dias, H. G. , Resck, M. E. B. , Caldas, G. C. , Resck, A. F. , Da Silva, N. V. , Dos Santos, A. M. V. , Sousa, T. D. C. , Ogrzewalska, M. H. , Siqueira, M. M. , Pauvolid‐Corrêa, A. , & Dos Santos, F. B. (2021). Neutralizing antibodies for SARS‐CoV‐2 in stray animals from Rio de Janeiro, Brazil. Plos One, 16(3), e0248578. 10.1371/journal.pone.0248578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten, C. , Günther, S. , Preiser, W. , Van Der Werf, S. , Brodt, H.‐R. , Becker, S. , Rabenau, H. , Panning, M. , Kolesnikova, L. , Fouchier, R. A. M. , Berger, A. , Burguière, A.‐M. , Cinatl, J. , Eickmann, M. , Escriou, N. , Grywna, K. , Kramme, S. , Manuguerra, J.‐C. , Müller, S. , … Doerr, H. W. (2003). Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New England Journal of Medicine, 348(20), 1967–1976. 10.1056/NEJMoa030747 [DOI] [PubMed] [Google Scholar]
- Ferasin, L. , Fritz, M. , Ferasin, H. , Becquart, P. , Legros, V. , & Leroy, E. M. (2021). Myocarditis in naturally infected pets with the British variant of COVID‐19. bioRxiv. 10.1101/2021.03.18.435945 [DOI]
- Fernández‐De‐Mera, I. G. , Rodríguez Del‐Río, F. J. , Fuente, J. , Pérez‐Sancho, M. , Hervás, D. , Moreno, I. , Domínguez, M. , Domínguez, L. , & Gortázar, C. (2020). Detection of environmental SARS‐CoV‐2 RNA in a high prevalence setting in Spain. Transboundary and Emerging Diseases, 68, 1487–1492. 10.1111/tbed.13817 [DOI] [PubMed] [Google Scholar]
- Fritz, M. , Rosolen, B. , Krafft, E. , Becquart, P. , Elguero, E. , Vratskikh, O. , Denolly, S. , Boson, B. , Vanhomwegen, J. , Gouilh, M. A. , Kodjo, A. , Chirouze, C. , Rosolen, S. G. , Legros, V. , & Leroy, E. M. (2020). High prevalence of SARS‐CoV‐2 antibodies in pets from COVID‐19+ households. One Health, 11, 100192. 10.1016/j.onehlt.2020.100192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gortázar, C. , Barroso‐Arévalo, S. , Ferreras‐Colino, E. , Isla, J. , De La Fuente, G. , Rivera, B. , Domínguez, L. , De La Fuente, J. , & Sánchez‐Vizcaíno, J. M. (2021). Natural SARS‐CoV‐2 infection in kept ferrets, Spain. Emerging Infectious Disease Journal, 27(7), 1994. 10.3201/eid2707.210096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, Y. , Zheng, B. J. , He, Y. Q. , Liu, X. L. , Zhuang, Z. X. , Cheung, C. L. , Luo, S. W. , Li, P. H. , Zhang, L. J. , Guan, Y. J. , Butt, K. M. , Wong, K. L. , Chan, K. W. , Lim, W. , Shortridge, K. F. , Yuen, K. Y. , Peiris, J. S. M. , & Poon, L. L. M. (2003). Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science, 302(5643), 276–278. 10.1126/science.1087139 [DOI] [PubMed] [Google Scholar]
- Haake, C. , Cook, S. , Pusterla, N. , & Murphy, B. (2020). Coronavirus infections in companion animals: Virology, epidemiology, clinical and pathologic features. Viruses, 12(9), 1023. 10.3390/v12091023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann, P. J. , Hatta, M. , Chiba, S. , Maemura, T. , Fan, S. , Takeda, M. , Kinoshita, N. , Hattori, S.‐I. , Sakai‐Tagawa, Y. , Iwatsuki‐Horimoto, K. , Imai, M. , & Kawaoka, Y. (2020). Transmission of SARS‐CoV‐2 in domestic cats. New England Journal of Medicine, 383(6), 592–594. 10.1056/NEJMc2013400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, S. A. , Pauvolid‐Corrêa, A. , Zecca, I. B. , Davila, E. , Auckland, L. D. , Roundy, C. M. , Tang, W. , Torchetti, M. , Killian, M. L. , Jenkins‐Moore, M. , Mozingo, K. , Akpalu, Y. , Ghai, R. R. , Spengler, J. R. , Behravesh, C. B. , Fischer, R. S. B. , & Hamer, G. L. (2020). Natural SARS‐CoV‐2 infections, including virus isolation, among serially tested cats and dogs in households with confirmed human COVID‐19 cases in Texas, USA. bioRxiv. 10.1101/2020.12.08.416339 [DOI] [PMC free article] [PubMed]
- Hammer, A. S. , Quaade, M. L. , Rasmussen, T. B. , Fonager, J. , Rasmussen, M. , Mundbjerg, K. , Lohse, L. , Strandbygaard, B. , Jørgensen, C. S. , Alfaro‐Núñez, A. , Rosenstierne, M. W. , Boklund, A. , Halasa, T. , Fomsgaard, A. , Belsham, G. J. , & Bøtner, A. (2021). SARS‐CoV‐2 transmission between mink (Neovison vison) and humans, Denmark. Emerging Infectious Disease Journal, 27(2), 547. 10.3201/eid2702.203794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs, E. C. , & Reid, T. J. (2020). Animals and SARS‐CoV‐2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transboundary and Emerging Diseases, 68, 1850–1867. 10.1111/tbed.13885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain, M. G. , Javed, A. , Akter, S. , & Saha, S. (2020). SARS‐CoV‐2 host diversity: An update of natural infections and experimental evidence. Journal of Microbiology, Immunology and Infection, 54, 175–181. 10.1016/j.jmii.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.‐I. , Kim, S.‐G. , Kim, S.‐M. , Kim, E.‐H. , Park, S.‐J. , Yu, K.‐M. , Chang, J.‐H. , Kim, E. J. , Lee, S. , Casel, M. A. B. , Um, J. , Song, M.‐S. , Jeong, H. W. , Lai, V. D. , Kim, Y. , Chin, B. S. , Park, J.‐S. , Chung, K.‐H. , Foo, S.‐S. , … Choi, Y. K. (2020). Infection and rapid transmission of SARS‐CoV‐2 in ferrets. Cell Host & Microbe, 27(5), 704–709. 10.1016/j.chom.2020.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , Li, M. , Knyaz, C. , & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547–1549. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassaunière, R. , Fonager, J. , Rasmussen, M. , Frische, A. , Strandh, C. P. , Rasmusse, T. B. , Bøtner, A. , & Fomsgaard, A. (2020). Working paper on SARS‐CoV‐2 spike mutations arising in Danish mink, their spread to humans and neutralization data.
- Lau, S. K. P. , Luk, H. K. H. , Wong, A. C. P. , Li, K. S. M. , Zhu, L. , He, Z. , Fung, J. , Chan, T. T. Y. , Fung, K. S. C. , & Woo, P. C. Y. (2020). Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerging Infectious Disease Journal, 26(7), 1542. 10.3201/eid2607.200092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T. , Liang, W. , Zhong, H. , He, J. , Chen, Z. , He, G. , Song, T. , Chen, S. , Wang, P. , Li, J. , Lan, Y. , Cheng, M. , Huang, J. , Niu, J. , Xia, L. , Xiao, J. , Hu, J. , Lin, L. , Huang, Q. , & Ma, W. (2020). Risk factors associated with COVID‐19 infection: A retrospective cohort study based on contacts tracing. Emerging Microbes & Infections, 9(1), 1546–1553. 10.1080/22221751.2020.1787799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig, S. , & Zarbock, A. (2020). Coronaviruses and SARS‐CoV‐2: A brief overview. Anesthesia and Analgesia, 131(1), 93–96. 10.1213/ane.0000000000004845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Guijosa, J. , Romero, B. , Infantes‐Lorenzo, J. A. , Díez, E. , Boadella, M. , Balseiro, A. , Veiga, M. , Navarro, D. , Moreno, I. , Ferreres, J. , Domínguez, M. , Fernández, C. , Domínguez, L. , & Gortázar, C. (2020). Environmental DNA: A promising factor for tuberculosis risk assessment in multi‐host settings. Plos One, 15(5), e0233837‐e0233837. 10.1371/journal.pone.0233837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . (2021). COVID‐19 portal, events in animals.
- Oude Munnink, B. B. , Sikkema, R. S. , Nieuwenhuijse, D. F. , Molenaar, R. J. , Munger, E. , Molenkamp, R. , Van Der Spek, A. , Tolsma, P. , Rietveld, A. , Brouwer, M. , Bouwmeester‐Vincken, N. , Harders, F. , Hakze‐Van Der Honing, R. , Wegdam‐Blans, M. C. A. , Bouwstra, R. J. , Geurtsvankessel, C. , Van Der Eijk, A. A. , Velkers, F. C. , Smit, L. A. M. , … Koopmans, M. P. G. (2021). Transmission of SARS‐CoV‐2 on mink farms between humans and mink and back to humans. Science, 371(6525), 172. 10.1126/science.abe5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paden, C. R. , Tao, Y. , Queen, K. , Zhang, J. , Li, Y. , Uehara, A. , & Tong, S. (2020). Rapid, sensitive, full‐genome sequencing of severe acute respiratory syndrome coronavirus 2. Emerging Infectious Diseases, 26(10), 2401–2405. 10.3201/eid2610.201800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh, R. , Mathai, A. , Parikh, S. , Chandra Sekhar, G. , & Thomas, R. (2008). Understanding and using sensitivity, specificity and predictive values. Indian Journal of Ophthalmology, 56(1), 45–50. 10.4103/0301-4738.37595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, E. I. , Elia, G. , Grassi, A. , Giordano, A. , Desario, C. , Medardo, M. , Smith, S. L. , Anderson, E. R. , Prince, T. , Patterson, G. T. , Lorusso, E. , Lucente, M. S. , Lanave, G. , Lauzi, S. , Bonfanti, U. , Stranieri, A. , Martella, V. , Solari Basano, F. , Barrs, V. R. , … Decaro, N. (2020). Evidence of exposure to SARS‐CoV‐2 in cats and dogs from households in Italy. Nature Communications, 11(1), 6231. 10.1038/s41467-020-20097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, R. A. P. M. , Ko, R. , Tsang, O. T. Y. , Hui, D. S. C. , Kwan, M. Y. M. , Brackman, C. J. , To, E. M. W. , Yen, H.‐L. , Leung, K. , Cheng, S. M. S. , Chan, K. H. , Chan, K. C. K. , Li, K.‐C. , Saif, L. , Barrs, V. R. , Wu, J. T. , Sit, T. H. C. , Poon, L. L. M. , & Peiris, M. (2021). Evaluation of a SARS‐CoV‐2 surrogate virus neutralization test for detection of antibody in human, canine, cat, and hamster sera. Journal of Clinical Microbiology, 59(2), e02504–e02520. 10.1128/JCM.02504-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken, C. B. , Haagmans, B. L. , Müller, M. A. , Gutierrez, C. , Godeke, G.‐J. , Meyer, B. , Muth, D. , Raj, V. S. , Vries, L. S.‐D. , Corman, V. M. , Drexler, J.‐F. , Smits, S. L. , El Tahir, Y. E. , De Sousa, R. , Van Beek, J. , Nowotny, N. , Van Maanen, K. , Hidalgo‐Hermoso, E. , Bosch, B.‐J. , … Koopmans, M. P. (2013). Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. The Lancet Infectious Diseases, 13(10), 859–866. 10.1016/S1473-3099(13)70164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, M. , Kok, A. , De Meulder, D. , Bestebroer, T. M. , Lamers, M. M. , Okba, N. M. A. , Fentener Van Vlissingen, M. , Rockx, B. , Haagmans, B. L. , Koopmans, M. P. G. , Fouchier, R. A. M. , & Herfst, S. (2020). SARS‐CoV‐2 is transmitted via contact and via the air between ferrets. Nature Communications, 11(1), 3496. 10.1038/s41467-020-17367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Arrondo, I. , Portillo, A. , Palomar, A. M. , Santibáñez, S. , Santibáñez, P. , Cervera, C. , & Oteo, J. A. (2020). Detection of SARS‐CoV‐2 in pets living with COVID‐19 owners diagnosed during the COVID‐19 lockdown in Spain: A case of an asymptomatic cat with SARS‐CoV‐2 in Europe. Transboundary and Emerging Diseases, 68, 973–976. 10.1111/tbed.13803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Wen, Z. , Zhong, G. , Yang, H. , Wang, C. , Huang, B. , Liu, R. , He, X. , Shuai, L. , Sun, Z. , Zhao, Y. , Liu, P. , Liang, L. , Cui, P. , Wang, J. , Zhang, X. , Guan, Y. , Tan, W. , Wu, G. , … Bu, Z. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS‐coronavirus 2. Science, 368(6494), 1016–1020. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia, S. F. , Yan, L.‐M. , Chin, A. W. H. , Fung, K. , Choy, K.‐T. , Wong, A. Y. L. , Kaewpreedee, P. , Perera, R. A. P. M. , Poon, L. L. M. , Nicholls, J. M. , Peiris, M. , & Yen, H.‐L. (2020). Pathogenesis and transmission of SARS‐CoV‐2 in golden hamsters. Nature, 583(7818), 834–838. 10.1038/s41586-020-2342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit, T. H. C. , Brackman, C. J. , Ip, S. M. , Tam, K. W. S. , Law, P. Y. T. , To, E. M. W. , Yu, V. Y. T. , Sims, L. D. , Tsang, D. N. C. , Chu, D. K. W. , Perera, R. A. P. M. , Poon, L. L. M. , & Peiris, M. (2020). Infection of dogs with SARS‐CoV‐2. Nature, 586(7831), 776–778. 10.1038/s41586-020-2334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. (1992). Estimation of the number of nucleotide substitutions when there are strong transition‐transversion and G+C‐content biases. Molecular Biology and Evolution, 9(4), 678–687. 10.1093/oxfordjournals.molbev.a040752 [DOI] [PubMed] [Google Scholar]
- Temmam, S. , Barbarino, A. , Maso, D. , Behillil, S. , Enouf, V. , Huon, C. , Jaraud, A. , Chevallier, L. , Backovic, M. , Pérot, P. , Verwaerde, P. , Tiret, L. , Van Der Werf, S. , & Eloit, M. (2020). Absence of SARS‐CoV‐2 infection in cats and dogs in close contact with a cluster of COVID‐19 patients in a veterinary campus. One Health, 10, 100164. 10.1016/j.onehlt.2020.100164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskarides, K. (2020). Animal‐to‐human viral transitions: Is SARS‐CoV‐2 an evolutionarily successful one? Journal of Molecular Evolution, 88(5), 421–423. 10.1007/s00239-020-09947-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Li, X. , Li, T. , Zhang, S. , Wang, L. , Wu, X. , & Liu, J. (2020). The genetic sequence, origin, and diagnosis of SARS‐CoV‐2. European Journal of Clinical Microbiology & Infectious Diseases, 39(9), 1629–1635. 10.1007/s10096-020-03899-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2020a). Origin of SARS‐CoV‐2.
- WHO . (2020b). SARS‐CoV‐2 mink‐associated variant strain – Denmark. Retrieved from https://www.who.int/csr/don/06-november-2020-mink-associated-sars-cov2-denmark/en/
- WHO . (2021). COVID‐19 Weekly Epidemiological Update ‐ 4 May 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-4-may-2021
- Wong, G. , Bi, Y. H. , Wang, Q. H. , Chen, X. W. , Zhang, Z. G. , & Yao, Y. G. (2020). Zoonotic origins of human coronavirus 2019 (HCoV‐19 /SARS‐CoV‐2): Why is this work important? Zoological Research, 41(3), 213–219. 10.24272/j.issn.2095-8137.2020.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki, A. M. , van Boheemen, S. , Bestebroer, T. M. , Osterhaus, A. D. , & Fouchier, R. A. (2012). Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New England Journal of Medicine, 367(19), 1814–1820. 10.1056/NEJMoa1211721 [DOI] [PubMed] [Google Scholar]
- Zhang, Q. , Zhang, H. , Huang, K. , Yang, Y. , Hui, X. , Gao, J. , He, X. , Li, C. , Gong, W. , Zhang, Y. , Peng, C. , Gao, X. , Chen, H. , Zou, Z. , Shi, Z. , & Jin, M. (2020). SARS‐CoV‐2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv. 10.1101/2020.04.01.021196 [DOI]
- Zhao, S. , Schuurman, N. , Li, W. , Wang, C. , Smit, L. A. M. , Broens, E. M. , Wagenaar, J. A. , Van Kuppeveld, F. J. M. , Bosch, B.‐J. , & Egberink, H. (2021). Serologic screening of severe acute respiratory syndrome coronavirus 2 infection in cats and dogs during first coronavirus disease wave, the Netherlands. Emerging Infectious Disease Journal, 27(5), 1362. 10.3201/eid2705.204055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Yang, Y. , Gao, J. , Huang, K. , Hu, C. , Hui, X. , He, X. , Li, C. , Gong, W. , Lv, C. , Zhang, Y. , Chen, H. , Zou, Z. , Zhang, Q. , & Jin, M. (2022). A serological survey of severe acute respiratory syndrome coronavirus 2 in in uhan. Transboundary and Emerging Diseases, 69, 591–597. 10.1111/tbed.14024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X.‐L. , Wang, X.‐G. , Hu, B. , Zhang, L. , Zhang, W. , Si, H.‐R. , Zhu, Y. , Li, B. , Huang, C.‐L. , Chen, H.‐D. , Chen, J. , Luo, Y. , Guo, H. , Jiang, R.‐D. , Liu, M.‐Q. , Chen, Y. , Shen, X.‐R. , Wang, X. , … Shi, Z.‐L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


