The SARS‐CoV‐2 entrance into the oral cavity occurs via the angiotensin‐converting enzyme 2 receptor (ACE2), which expression has been found in salivary gland ductal epithelium (Liu et al., 2011), tongue (Xu et al., 2020), and periodontal tissue (Fernandes Matuck et al., 2020). Hence, cells with ACE2 receptors may turn into host cells for SARS‐CoV‐2 and be related to oral manifestations of COVID‐19 and inflammatory response in oral sites (Xu et al., 2020). Preliminary studies have reported oral manifestations possibly related to COVID‐19 (Amorim Dos Santos et al., 2020; Ansari et al., 2020; Martín Carreras‐Presas et al., 2020). Clinical manifestation showing multiple ulcerative lesions seems to be the most common feature. Some reports related cases suspicious of COVID‐19 but had no confirmed diagnosis of SARS‐CoV‐2 infection and we believe this might have biased the results (Martín Carreras‐Presas et al., 2020). The authors do not exclude that the effects of stress may be a trigger for the development of oral lesions, as it is known as a possible causal factor in this kind of conditions (Gallo et al., 2009).
We present a clinical–pathological report on autopsies of patients who died of COVID‐19 in order to elucidate the possibility of oral manifestations of COVID‐19 as there have been some divergences in the literature regarding the oral outcome.
Twenty‐six deceased patients (15 females and 11 males) with SARS‐CoV‐2 were submitted to oral examination during the minimally invasive autopsy procedures (Duarte‐Neto et al., 2019). All patients had severe acute respiratory syndrome and were admitted in the ICU of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo for mechanical ventilation support. Only SARS‐CoV‐2 positive cases were included. SARS‐CoV‐2 tests were performed during hospital admission or ICU stay by using RT‐PCR in nasopharyngeal swabs or bronchoalveolar secretion. The mean age was 50 years old (8–76 years), the average number of days of hospitalization was 15.30 days (3–40 days), and the average number of days under mechanical ventilation in the ICU was 12.36 days (0–43 days). The mean time between diagnosis and death was 10.55 days, and the mean time between death and autopsy was 16.95 h (5–22 h). Twenty‐four patients were non‐ or ex‐smokers, and only two patients had smoking habits (narghile and marijuana). Of the 26 patients, ten (38.46%) were free from comorbidities and the others had at least one disease (i.e., hypertension or diabetes).
A dentist and an otorhinolaryngologist examined the intraoral and perioral sites in the autopsy. Oral evaluation included hard palate, tongue, jugal and gingival mucosa, anterior tonsillar pilar, and inner lips. Biopsies were performed with scalpels and punch, resulting in multiple fragments as follows: three fragments of the tongue (i.e., apex, lateral, and posterior), two gingival fragments of periodontal tissues, one fragment of the amygdala, and one fragment of the inner lip mucosa. Patients who had some oral lesions diagnosed during clinical evaluation; biopsies were performed in the lesion site. Tissue specimens were fixed in buffered 10% formalin and embedded in paraffin, sections were cut and stained with hematoxylin and eosin (H&E). Immunohistochemical staining was performed using the polyclonal Anti‐HSV1 Abcam (Ab9533), monoclonal Anti‐Cytomegalovirus glycoprotein B (Abcam17073).
Patients 1 and 2 had ulcerative lesions in the lower lip and one in the tongue as well. Both patients were edentulous and stayed in the ICU under mechanical ventilation for 10 and 25 days, respectively. Biopsy was performed and histopathological analysis showed diffuse infiltration of inflammatory mononuclear cells, which validates our clinical findings of traumatic injuries resulting from the use of orotracheal tubes for mechanical ventilation support (Figure 2).
FIGURE 2.
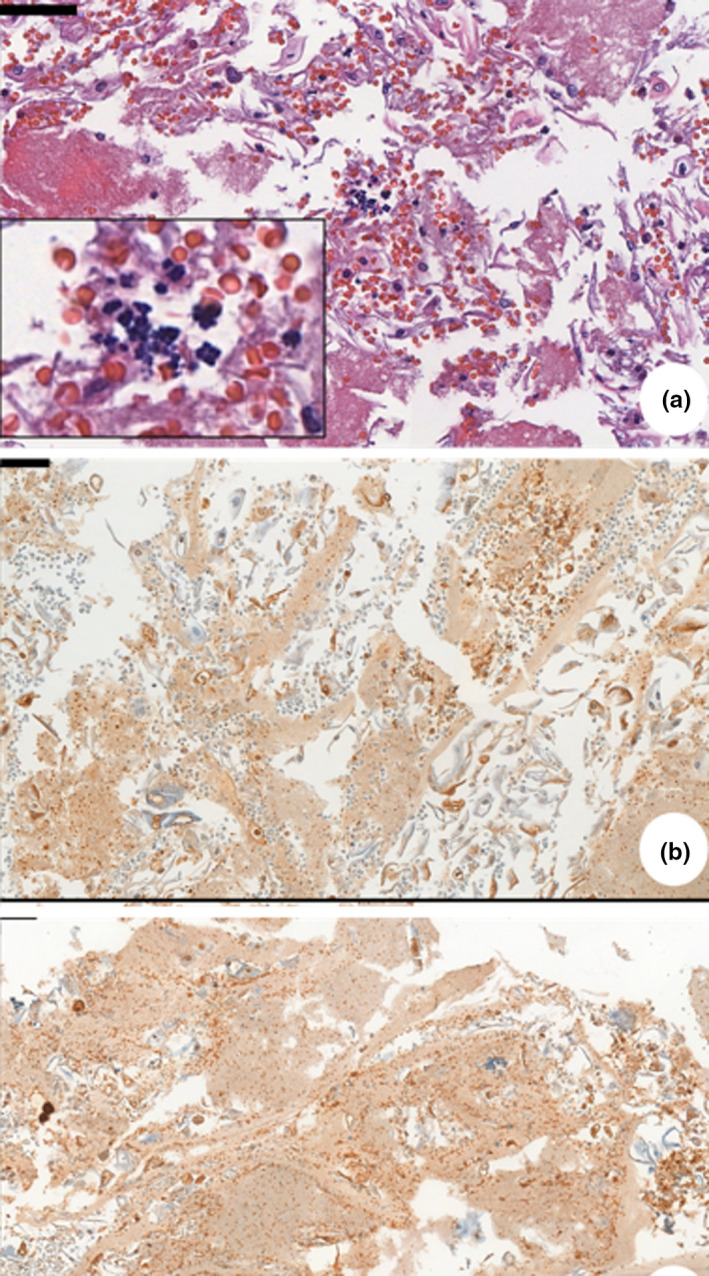
(a) Photomicrograph of a cell block of patient pictured in Figure 1h—routine hematoxylin and eosin (HE) stain. Sample of an ulcerative lesion in the lower lip. Sarcina ventriculi cocci characterized by multiple gram‐positive basophilic colored tetrad arrangement, scattered in epithelium. (b) Immunohistochemical of a cell block—anti‐HSV1 (abcam9533), Sample of an ulcerative lesion on dorsal tongue and buccal mucosa. Immunopositivity seen inside epithelial cell from basal layer
Patient 3 and 4 showed vesiculo‐bullous and ulcerative lesions scattered in the oral mucosa, such as tongue and jugal mucosa. Immunohistochemical staining was performed and indicated the presence of herpes simplex virus (HSV‐1) in both cases (Figure 2). It is known that critically ill patients have great potential of developing reactivation of some herpesviruses due to ICU and long‐term hospitalization (Simonnet et al., 2021). Patient 3 still showed co‐infection by fungal forms compatible with Candida spp (spores, pseudohyphae, and hyphae).
Patient 5 presented ulcerative lesions, and the histopathological analysis showed co‐infection by typical Sarcina ventriculi colonies (Figure 2). S. ventriculi is a gram‐positive coco that usually appears in gastric biopsies with late gastric emptying and your presence may indicate a gastric pathology (Al Rasheed & Senseng, 2016). Severely ill or immunocompromised patients have a higher probability of suffering from invasive fungal and bacterial co‐infections and these findings are consistent with previous reports (Salehi et al., 2020).
Integrity of oral mucosa was preserved in all other cases (Figure 1). No other patient examined had atypical oral manifestations, such as vesiculo‐bullous lesions or ulcerations in the keratinized epithelium. Histopathological features of the cases with no clinical manifestation in the tongue, gingival papilla, inner lip mucosa and anterior tonsillar pillar showed some morphological alterations in the keratinocytes of the lining and junctional epithelium, characterized mainly by vacuolization of the cytoplasm and nucleus, and sometimes nuclear pleomorphism. Infiltration of inflammatory mononuclear or polymorphonuclear cells was rarely observed, even in cases of severe oral deterioration (Figure 2).
FIGURE 1.
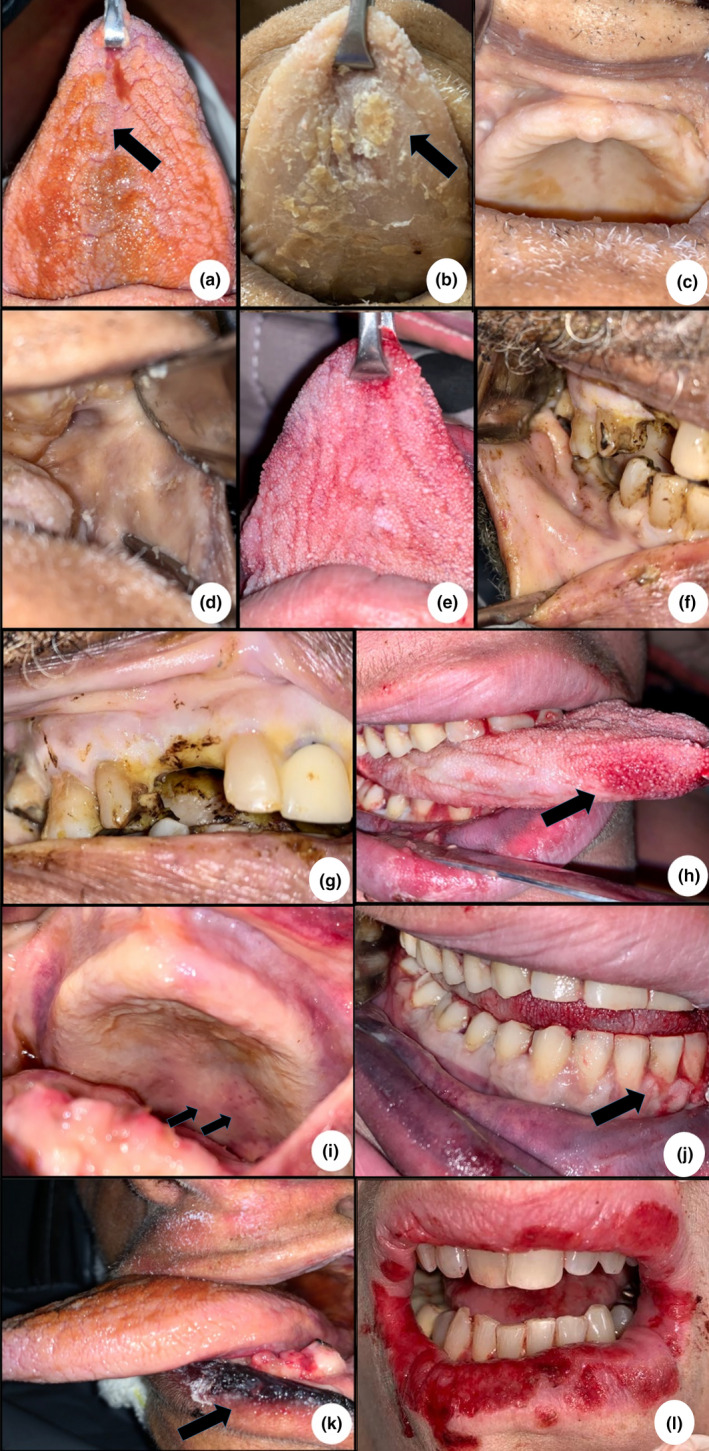
Clinical aspects of oral sites on deceased patients of COVID‐19. (a, b) Epithelial desquamation due to the shock, masticatory mucosa integrity preserved. (c–e) Patients showing no alterations on mucosa integrity. (f, g) Presence of oral health deterioration with dental caries and missing teeth. (h–j) Ulcerative lesion—Inset showing erythematous lesions in lateral tongue, palate and gingiva. (k) Large ulcerative lesions on lower lip due to trauma caused by long‐term use of orotracheal tube. (h) Ulcerative lesions in upper and lower lip, extending to gingiva and tongue Scale bars: 50 μm
The oral manifestations found in our patients and those reported in other studies corroborate the hypothesis of secondary oral lesions induced by co‐infection and immune impairment possibly occurring as an adverse reaction from the therapeutic and support measures for COVID‐19 (Amorim Dos Santos et al., 2020, 2021; Martín Carreras‐Presas et al., 2020; Putra et al., 2020).
The absence of oral lesions related to SARS‐CoV‐2 in our case series did not exclude the possibility of viral presence in oral tissues. Once the new virus has this distinct organotropism, it is rationale that physicians and dentists try to look for a link between a new disease and oral findings (Puelles et al., 2020). Histopathological, immunohistochemical, and biomolecular studies are the gold standard for understanding the cause‐effect relations and therefore are essential to a correct link between oral manifestations and SARS‐CoV‐2 infection.
Patients in ICU are susceptible to lesions resulting from mechanical ventilation, enteral feeding, orotracheal tubes, and health deterioration (Dziedzic & Wojtyczka, 2020). Therefore, clinical and physical examinations are highly recommended and indispensable during hospitalization time and sometimes must include biopsies when needed. Biopsies are an important tool to relate clinical conditions to etiological agents. Other studies, mainly those on pulmonary manifestations, are trying to find a correlation between histopathological features and clinical conditions (Duarte‐Neto et al., 2020). In cutaneous manifestations, histological evaluation showed non‐specific inflammatory infiltration, which is consistent with other viral exanthemas (Amatore et al., 2020). In our study, we performed postmortem biopsy in some oral sites due to the presence of some inflammatory activity, and we found in some cases the presence of herpes simplex virus. In situ evaluations should be considered (e.g., hybridization or immunohistochemistry to SARS‐CoV‐2) to know whether these lesions are related to external conditions or a cytopathic effect.
The oral manifestations of COVID‐19 found in our postmortem case report are secondary lesions related to trauma events, co‐infections involving bacterium, Candida spp, Sarcinia ventriculi, herpes simplex virus, immune impairment due to disease itself and adverse reactions from the therapeutic interventions. Most of the deceased patients evaluated had long‐term hospitalization, received mechanical ventilation support and some developed oral injuries during hospital stay. Different from what the literature suggested in the beginning of the pandemic, oral lesions in severe cases of COVID‐19 infected patients seem not to be related specifically to SARS‐CoV‐2 or due to a cytopathic event that effects oral mucosa.
CONFLICT OF INTEREST
All authors of this work declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Amanda Zarpellon: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Resources; Validation; Visualization; Writing‐original draft; Writing‐review & editing. Bruno Matuck: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing‐original draft; Writing‐review & editing. Marisa Dolhnikoff: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing‐review & editing. Amaro Nunes Duarte‐Neto: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Visualization; Writing‐original draft; Writing‐review & editing. Gilvan Maia: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing‐review & editing. Sara Costa Gomes: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing‐review & editing. Daniel Isaac Sendyk: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing‐review & editing. Suzana Machado Sousa: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing‐original draft; Writing‐review & editing. Thais Mauad: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Visualization; Writing‐review & editing. Paulo Hilário do Nascimento Saldiva: Conceptualization; Data curation; Formal analysis; Project administration; Supervision; Validation; Visualization; Writing‐review & editing. Paulo Henrique Braz‐Silva: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing‐original draft; Writing‐review & editing. Luiz Fernando Ferraz Silva: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing‐original draft; Writing‐review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/odi.14047.
ACKNOWLEDGEMENTS
The authors wish to thank Kely Cristina Soares Bispo, Gustavo Linari Rodrigues, Angela B G dos Santos, Sandra de Moraes Fernezlian, Reginaldo Silva do Nascimento, Glaucia Aparecida dos Santos Bento, Thábata Larissa, Luciano Ferreira Leite, and Catia Sales de Moura for their technical support. We would also to acknowledge all health care providers involved in the care of the patients with COVID‐19 and all Hospital (HC‐FMUSP) and São Paulo Autopsy Service staff who have taken part in the Coronavirus Crisis Task Force during the epidemic season. We acknowledge and we are deeply thankful to all relatives and legal representatives who have consented the postmortem examinations of their beloved relatives lost to the COVID‐19.
Zarpellon, A. , Matuck, B. F. , Dolhnikoff, M. , Duarte‐Neto, A. N. , Maia, G. , Gomes, S. C. , Sendyk, D. I. , Souza, S. C. O. M. , Mauad, T. , Saldiva, P. H. N. , Braz‐Silva, P. H. , & da Silva, L. F. F. (2021). Oral lesions and SARS‐CoV‐2: A postmortem study. Oral Diseases, 00, 1–5. 10.1111/odi.14047
Amanda Zarpellon and Bruno Fernandes Matuck contributed equally to the work.
Funding information
Fundação de Amparo à Pesquisa do Estado de São Paulo 2013/17159‐2; Bill and Melinda Gates Foundation INV‐002396; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) 401825/2020‐5; Pró‐Reitoria de Pesquisa da Universidade de São Paulo 2021.1.10424.1.9
REFERENCES
- Al Rasheed, M. R. , & Senseng, C. G. (2016). Sarcina ventriculi: Review of the literature. Archives of Pathology and Laboratory Medicine, 140(12), 1441–1445. 10.5858/arpa.2016-0028-RS [DOI] [PubMed] [Google Scholar]
- Amatore, F. , Macagno, N. , Mailhe, M. , Demarez, B. , Gaudy‐Marqueste, C. , Grob, J. J. , Raoult, D. , Brouqui, P. , & Richard, M. A. (2020). SARS‐CoV‐2 infection presenting as a febrile rash. Journal of the European Academy of Dermatology and Venereology, 34(7), e304–e306. 10.1111/jdv.16528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim dos Santos, J. , Normando, A. , Carvalho da Silva, R. L. , Acevedo, A. C. , De Luca Canto, G. , Sugaya, N. , Santos‐Silva, A. R. , & Guerra, E. (2021). Oral manifestations in patients with COVID‐19: A living systematic review. Journal of Dental Research, 100(2), 141–154. 10.1177/0022034520957289 [DOI] [PubMed] [Google Scholar]
- Amorim Dos Santos, J. , Normando, A. G. C. , Carvalho da Silva, R. L. , De Paula, R. M. , Cembranel, A. C. , Santos‐Silva, A. R. , & Guerra, E. N. S. (2020). Oral mucosal lesions in a COVID‐19 patient: New signs or secondary manifestations? International Journal of Infectious Diseases, 97, 326–328. 10.1016/j.ijid.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari, R. , Gheitani, M. , Heidari, F. , & Heidari, F. (2020). Oral cavity lesions as a manifestation of the novel virus (COVID‐19): A letter‐to‐editor. Oral Diseases, 27, 771–772. 10.1111/odi.13465 [DOI] [PubMed] [Google Scholar]
- Duarte‐Neto, A. N. , Monteiro, R. A. D. A. , Johnsson, J. , Cunha, M. D. P. , Pour, S. Z. , Saraiva, A. C. , Ho, Y.‐L. , da Silva, L. F. F. , Mauad, T. , Zanotto, P. M. D. A. , Saldiva, P. H. N. , de Oliveira, I. R. S. , & Dolhnikoff, M. (2019). Ultrasound‐guided minimally invasive autopsy as a tool for rapid post‐mortem diagnosis in the 2018 Sao Paulo yellow fever epidemic: Correlation with conventional autopsy. PLoS Neglected Tropical Diseases, 13(7), e0007625. 10.1371/journal.pntd.0007625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte‐Neto, A. N. , Monteiro, R. A. A. , Silva, L. F. F. , Malheiros, D. M. A. C. , Oliveira, E. P. , Theodoro‐Filho, J. , Pinho, J. R. R. , Gomes‐Gouvêa, M. S. , Salles, A. P. M. , Oliveira, I. R. S. , Mauad, T. , Saldiva, P. H. N. , & Dolhnikoff, M. (2020). Pulmonary and systemic involvement in COVID‐19 patients assessed with ultrasound‐guided minimally invasive autopsy. Histopathology, 77(2), 186–197. 10.1111/his.14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzic, A. , & Wojtyczka, R. (2020). The impact of coronavirus infectious disease 19 (COVID‐19) on oral health. Oral Diseases, 27, 703–706. 10.1111/odi.13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Matuck, B. , Dolhnikoff, M. , Maia, G. V. A. , Isaac Sendyk, D. , Zarpellon, A. , Costa Gomes, S. , Duarte‐Neto, A. N. , Rebello Pinho, J. R. , Gomes‐Gouvêa, M. S. , Sousa, S. C. O. M. , Mauad, T. , Saldiva, P. H. D. N. , Braz‐Silva, P. H. , & Silva, L. F. F. D. (2020). Periodontal tissues are targets for Sars‐Cov‐2: A post‐mortem study. Journal of Oral Microbiology, 13(1), 1848135. 10.1080/20002297.2020.1848135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo, C. D. B. , Mimura, M. A. M. , & Sugaya, N. N. (2009). Psychological stress and recurrent aphthous stomatitis. Clinics (Sao Paulo), 64(7), 645–648. 10.1590/S1807-59322009000700007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Wei, Q. , Alvarez, X. , Wang, H. , Du, Y. , Zhu, H. , Jiang, H. , Zhou, J. , Lam, P. , Zhang, L. , Lackner, A. , Qin, C. , & Chen, Z. (2011). Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. Journal of Virology, 85(8), 4025–4030. 10.1128/JVI.02292-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín Carreras‐Presas, C. , Amaro Sánchez, J. , López‐Sánchez, A. F. , Jané‐Salas, E. , & Somacarrera Pérez, M. L. (2020). Oral vesiculobullous lesions associated with SARS‐CoV‐2 infection. Oral Diseases, 27(S3), 710–712. 10.1111/odi.13382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles, V. G. , Lütgehetmann, M. , Lindenmeyer, M. T. , Sperhake, J. P. , Wong, M. N. , Allweiss, L. , Chilla, S. , Heinemann, A. , Wanner, N. , Liu, S. , Braun, F. , Lu, S. , Pfefferle, S. , Schröder, A. S. , Edler, C. , Gross, O. , Glatzel, M. , Wichmann, D. , Wiech, T. , … Huber, T. B. (2020). Multiorgan and Renal Tropism of SARS‐CoV‐2. New England Journal of Medicine, 383(6), 590–592. 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putra, B. E. , Adiarto, S. , Dewayanti, S. R. , & Juzar, D. A. (2020). Viral exanthem with "Spins and needles sensation" on extremities of a COVID‐19 patient: A self‐reported case from an Indonesian medical frontliner. International Journal of Infectious Diseases, 96, 355–358. 10.1016/j.ijid.2020.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi, M. , Ahmadikia, K. , Mahmoudi, S. , Kalantari, S. , Jamalimoghadamsiahkali, S. , Izadi, A. , Kord, M. , Dehghan Manshadi, S. A. , Seifi, A. , Ghiasvand, F. , Khajavirad, N. , Ebrahimi, S. , Koohfar, A. , Boekhout, T. , & Khodavaisy, S. (2020). Oropharyngeal candidiasis in hospitalised COVID‐19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses, 63(8), 771–778. 10.1111/myc.13137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet, A. , Engelmann, I. , Moreau, A.‐S. , Garcia, B. , Six, S. , El Kalioubie, A. , Robriquet, L. , Hober, D. , & Jourdain, M. (2021). High incidence of Epstein‐Barr virus, cytomegalovirus, and human‐herpes virus‐6 reactivations in critically ill patients with COVID‐19. Infectious Diseases Now, 51(3), 296–299. 10.1016/j.idnow.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H. , Zhong, L. , Deng, J. , Peng, J. , Dan, H. , Zeng, X. , Li, T. , & Chen, Q. (2020). High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science, 12(1), 8. 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]


