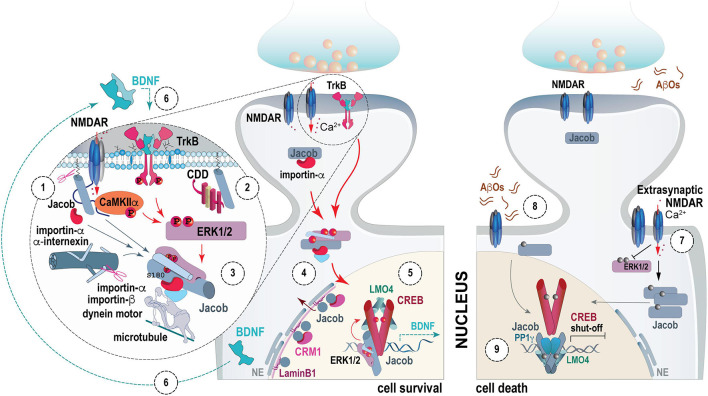
FIGURE 1.
Molecular mechanisms underlying synapse-to-nucleus trafficking of Jacob and its nuclear function. N-terminal myristoylation is a prerequisite for the extranuclear localization of Jacob. In synapses, Jacob associates with the GluN2B-containing NMDA receptor complex as well as CaMKII-α (1). The synaptic localization is regulated by the neuronal Ca2+ sensor protein Caldendrin (2) that competes with importins for Jacob binding. NMDAR activation leads to calpain-mediated cleavage of the myristoylated part of the protein and releases Jacob from the plasma membrane. Concomitantly, synaptic GluN2B-containing NMDAR activity leads to CaMKII-α dependent activation of ERK1/2, subsequent phosphorylation of Jacob at S180 and formation of a stable trimeric complex between phosphorylated Jacob, active ERK1/2 and the proteolytically cleaved fragment of the neuronal filament α-internexin (3) which protects pJacob and pERK1/2 against phosphatase activity during retrograde transport to the nucleus but likely also in the nucleus. Long-distance transport of the Jacob signalosome involves its association with importin-α, importin-β, and, subsequently the molecular motor dynein that moves along microtubules in a retrograde direction. GluN2B-containing NMDA receptor activity mediates the association of Jacob with the inner nuclear membrane where it transiently binds to LaminB1 (4). The association with the canonical CRM1-RanGTP-dependent export complex defines its nuclear residing time. In the nucleus, the Jacob signalosome associates with the CREB complex and results in its sustained activation by docking the active ERK1/2 in its close vicinity (5). This, in turn, promotes CREB-dependent gene expression of plasticity-related genes like Bdnf. In early development, BDNF induces the nuclear accumulation of phosphorylated Jacob in an NMDAR-dependent manner, which results in increased phosphorylation of CREB and enhanced CREB-dependent Bdnf gene expression in a positive feedback loop (6). Activation of extrasynaptic NMDARs by NMDA (7) or AβOs (8) does not lead to phosphorylation of ERK1/2 or Jacob. Nevertheless, the non-phosphorylated protein translocates to the nucleus (9) piggyback with the CREB phosphatase, PP1. In addition, it displaces CREB from the transcriptional co-activator LMO4 leading to CREB shut-off. CDD, Caldendrin; NE, nuclear envelope; red circle, phosphorylation; gray circle, unphosphorylated site; TrkB, Tropomyosin receptor kinase B; NMDAR, N-methyl-D-aspartate receptor; LMO4, LIM domain only 4; CREB, cAMP response element-binding protein; BDNF, brain-derived neurotrophic factor; CRM1, chromosomal maintenance 1; ERK1/2, Extracellular signal-regulated protein kinases 1 and 2; PP1, protein phosphatase 1. Scissors indicate cleavage.