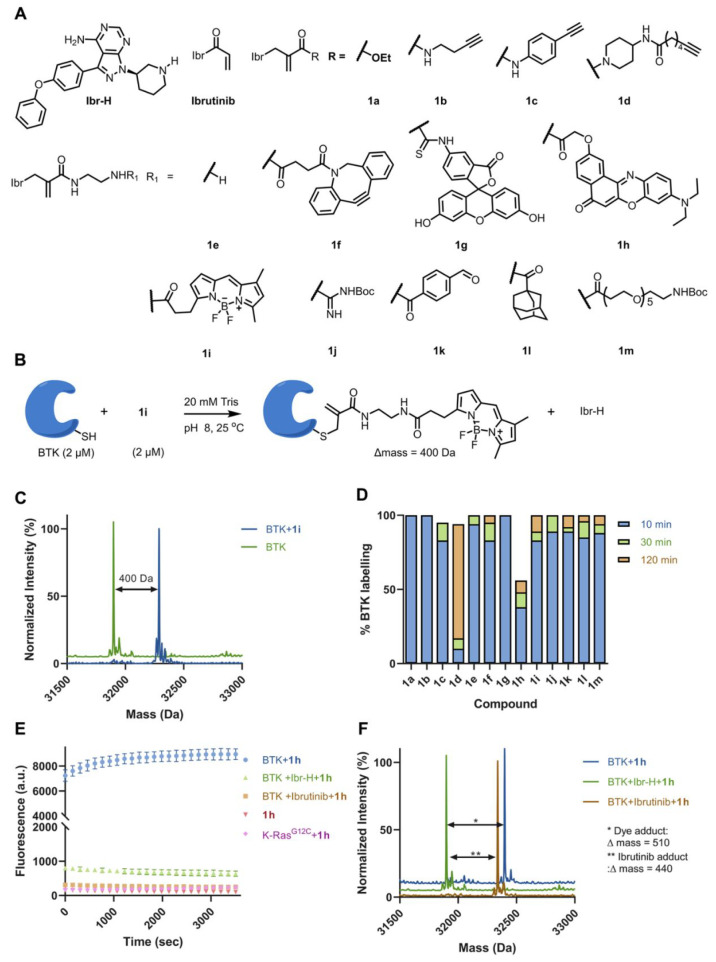
Figure 2.
Site-selective labeling of BTK using CoLDR chemistry. (A) Chemical structures of ibrutinib-directed methacrylamides with various functional tags. (B) Typical example of the reaction of BTK (2 μM) with 1i (2 μM) in a 20 mM Tris buffer at pH 8, 25 °C. (C) Deconvoluted LC/MS spectrum shows the labeling of a BODIPY probe and demonstrates Ibr-H leaving. (D) Percent of labeling of BTK (2 μM) with the probes (1a–1m; 2 μM) at 10, 30, and 120 min in 20 mM Tris buffer at pH 8, 25 °C. (E) Kinetics of the increase in fluorescence intensity measured at Ex/Em = 550/620 nm (n = 4) upon addition of BTK (2 μM) to 1h (2 μM) in 20 mM Tris buffer at pH 8, 37 °C (blue). Control experiments without BTK (red), preincubation of ibrutinib (4 μM) and Ibr-H (4 μM) prior to adding 1h (green and orange, respectively), and incubation of K-RasG12C (pink) with 1h show no fluorescence. (F) Deconvoluted LC/MS spectra for BTK incubated with 1h at the end of the fluorescence measurement (shown in E). The adduct mass corresponds to a labeling event in which the Ibr-H moiety was released, validating the proposed mechanism.