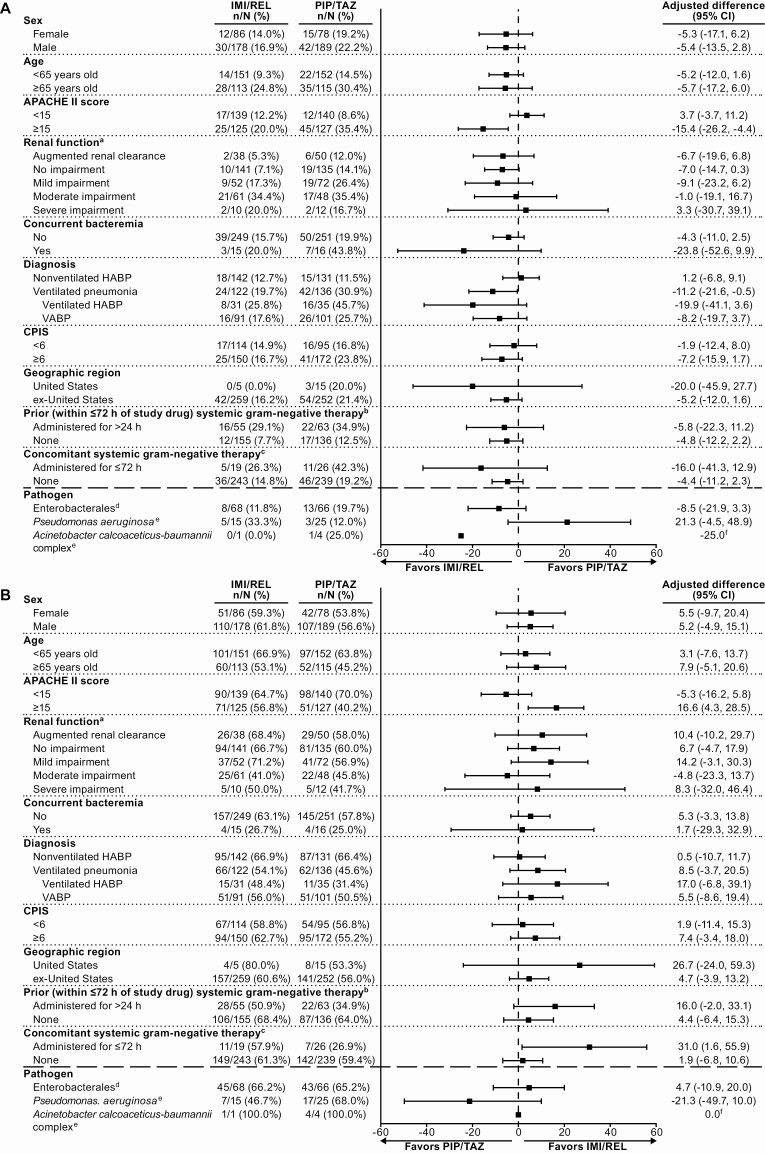
Figure 2.
Primary and key secondary efficacy endpoints in clinically relevant patient subgroups of the modified intent-to-treat (MITT) population: 28-day all-cause mortality (A) and favorable clinical response (B). (Note: Per-pathogen outcomes are shown for microbiologic modified intent-to-treat [mMITT] patients with all baseline lower respiratory tract [LRT] isolates susceptible to both study drugs.) aPost hoc analysis; all other subgroups were prospectively defined. bOutcomes in patients who received <24 hours of prior, systemic, gram-negative therapy (applicable to 20.5% of imipenem/cilastatin with relebactam [IMI/REL] and 25.5% of piperacillin/tazobactam [PIP/TAZ] patients) are not shown. cTwo patients in each treatment arm received >72 hours of concomitant, systemic, gram-negative therapy; outcomes in this very small subpopulation are not shown. dOutcomes are shown for the subpopulation of mMITT patients with only Enterobacterales (of any species) baseline LRT isolates and all baseline isolates susceptible to both IMI/REL and PIP/TAZ. eOutcomes are shown for the subpopulation of mMITT patients with ≥1 baseline LRT isolate of this pathogen and all baseline isolates susceptible to both IMI/REL and PIP/TAZ. fCIs were not calculated due to the low sample size (<5 patients in both arms) of this subpopulation. Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; CI, confidence interval; CPIS, Clinical Pulmonary Infection Score; HABP, hospital-acquired bacterial pneumonia; IMI/REL, imipenem/cilastatin with relebactam; PIP/TAZ, piperacillin/tazobactam; VABP, ventilator-associated bacterial pneumonia.