| Characteristic | Description/values | Method (when relevant) |
|---|---|---|
| Name |
Iron oxo‐hydroxide adipate tartrate IHAT |
n.a. |
| Description | IHAT is a tartrate‐modified, nano‐disperse Fe(III) oxohydroxide, formed in an adipate buffer, with similar functional properties and primary particle size as the iron found in the ferritin core. The tartrate‐modified ferrihydrite is precipitated from an Fe(III) chloride solution in the presence of sodium tartrate and adipate buffer. Fe(III)‐oxohydroxide nanocores are constrained from growth and crystallisation by being captured inside a corona of tartrate with some dispersion‐aiding adipic acid and tartaric acid mixed into the formulation. | ICP‐OES TEM and EDX XRD, STEM, FTIR, and EELS (see Powell et al., 2014) |
| Intended use | Dietary source of iron | n.a. |
| Material composition |
Hydrated matter: iron 28.0 (25.2–31.2) w/w %, tartaric acid 28.2 (26.7–31.0) w/w %, adipic acid 1.8 (1.7–2.1) w/w %, sodium 9.0 (8.7–9.7) w/w %, w/w %, chloride 3.1 (2.3–3.7) w/w %, and adsorbed water 15.9 (11.2–20.7) w/w %. The remainder (14%) is assumed to be structural hydrogen and oxygen, as per the accepted structure for 2‐line ferrihydrite (5Fe2O3 8H2O; Chappell et al., 2017). As dry matter: iron 33.2 (30.4–35.1) w/w %, tartaric acid 32.7 (28.9–35.0) w/w %, adipic acid 2.1 (1.9‐2.5) w/w %, sodium 10.7 (10.5–11.0) w/w %, chloride 3.6 (2.6–4.1) w/w %. |
Iron: ICP‐OES, UV.VIS Adipic and tartaric acids: HPLC‐DAD Sodium, chloride: ICP‐OES Water: Karl Fischer titration |
| Elemental composition | Primary nanoparticles have been shown to contain iron, oxygen, and carbon. | EDX |
| CAS number | 2460638‐28‐0 | n.a. |
| Molecular weight |
Average molecular weight: 35,803.4 Da (lower limit: 27,670.5 Da; upper limit: 45,319.4 Da). These results were obtained by modelling. The model was based on:
|
Modelled |
| Molecular formula |
FeOm(OH)n(H2O)x(C4H6O6)y(C 6 H 10 O 4 )z where: m and n are undefined as per accepted practice for ferric iron oxohydroxides (see Cornell and Schwertmann, 2003) x = 0.28–0.88 y = 0.78–1.50 z = 0.04–0.19 Tartaric acid (C4H6O6) and adipic acid (C6H10O4) are represented in their protonated form. |
Calculated |
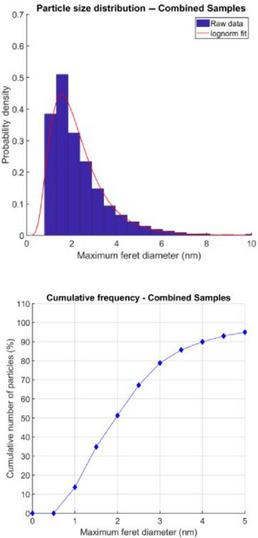
| Constituent particle size |
Minimum external dimension by electron microscopy: Median diameter = 1.98 nm (uncertainty = 0.02 nm, 95% confidence level), width of distribution: mean absolute value (MAD) = 0.99 nm;
Mean diameter = 2.31 nm (uncertainty = 0.02 nm, 95% confidence level), width of distribution: standard deviation (SD) = 1.39 nm. |
Electron microscopy (HAADF‐STEM) |
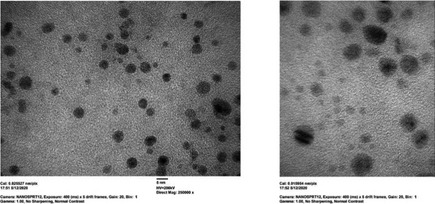
| Particle shape |
Constituent particles of almost spherical shape. TEM micrographs at magnifications: (a) 250,000x (b) 400,000x. |
TEM |
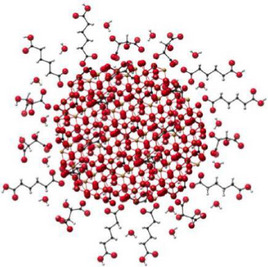
| Structure |
Schematic molecular structure of IHAT (red: oxygen; white: hydrogen; black: carbon; brown: iron) |
n.a. |
| Specific surface area | Given that the median radius of IHAT particles is 1.55 nm (volume mean diameter 3.1 nm) and the density is 2.15 g/cm3, a value of 904 m2/g is obtained. | Calculated |
| Appearance | Red‐brown microsized powder. | n.a. |
| Density | 2.14 g/cm3 | Pycnometry |
| Surface charge | Zeta potential at pH 6.4: – 47.6 mV. | Electrophoretic light scattering |
| Solubility [g/L] | 2–4% in water (proportion of solute in solvent at room temperature, with regard to iron content). | n.a. |
| Agglomeration and/or aggregation state and size |
In water, the following phase distribution is observed by ultrafiltration and ICP‐OES analysis: 95.9% nanoparticulates, 2.8% soluble, 1.3% microparticulates. Nanoparticulates show the following size distribution by dynamic light scattering expressed as a function of volume (Dv nm; conversion from intensity of the scattering signal to volume [mean ± SD]): Dv(10): 1.9 ± 0.24 Dv(50): 3.1 ± 0.25 Dv(90): 5.4 ± 0.32 (refractive Index 1.920, Absorption = 0.10). Microparticulates show the following particle size distribution by laser diffraction (volume‐based particle size; µm [mean ± SD]): Dv(10): 26 ± 33.08 Dv(50): 547.7 ± 67.20 Dv(90): 1,286.6 ± 52.73. |
Ultrafiltration and ICP‐OES Dynamic light scattering Laser diffraction |

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.