Abstract
Background
Ultrasound guided tenotomy (USGT) is a minimally invasive treatment option for patients with chronic tendinopathy. There are conflicting findings in the literature with some studies reporting severe complications and others reporting none. This variability is likely due to the small sample sizes of previous studies. We aimed to evaluate the risks associated with USGT and outcomes across multiple tendinopathy/fasciopathy sites in a large clinical sample.
Methods
Patients who had USGT were identified by retrospective review of charts. Complications, satisfaction, and outcomes (pain, quality of life) were assessed at baseline prior to the procedure (outcomes only), short-term follow up, and long term follow up.
Results
A total of 262 patients with 289 procedures were identified through chart review. There was a low complication rate of 0.7% including one superficial wound infection and one case of wound hypersensitivity. The majority of patients reported improvement in pain by short-term and long-term follow-up and improvement in function by long-term follow-up. The majority of responders reported being either ‘very satisfied’ or ‘somewhat satisfied’ with the procedure at short-term follow-up.
Conclusion
This study found that USGT is a safe procedure with a low complication rate in a heterogeneous sample. Study findings provide preliminary evidence on the utility of USGT to reduce pain and improve function with a high rate of patient satisfaction.
Level of Evidence: IV
Keywords: enthesopathy, tendinopathy, tenotomy, debridement
Introduction
Tendinopathy is a clinical syndrome characterized by pain and dysfunction related to mechanical loading of a tendon.1 Multiple treatments have been reported to alleviate pain and improve function, but short and long-term outcome data are limited. Historically, tendinopathy treatment has focused on activity modification and rehabilitative exercise interventions with progression to surgery when conservative treatments have failed.2 Minimally invasive ultrasound guided procedures are now becoming an option when surgical management is being considered. Ultrasound guided tenotomy and debridement (USGT) allows patients to return to normal activity sooner than traditional surgical procedures and may have less risk of complication. However, the current literature on USGT remains limited necessitating a higher level of evidence to understand the risks and effectiveness of this procedure.
There is currently a divide in the literature with 2 case series reporting serious complications due to USGT while other small studies report 0 complications. In particular for the Achilles tendon, a case series reported complications related to 6 procedures performed on the Achilles tendon including deep vein thrombosis (n = 1) and increased pain or no improvement in pain post-procedure (n = 5).3 Another case series described 2 procedures that resulted in 6-week post-operative rupture of the Achilles tendon.4 In contrast, another study on USGT for 25 patients with Achilles tendinopathy reported 0 complications and a success rate of 84% of patients with less pain and activity limitation.5 Similarly, we previously reported that patients with Achilles tendinopathy had reduced pain and high patient satisfaction after USGT and the only complication out of 40 procedures was a superficial skin infection.6 Moreover, the majority of data on USGT has focused on elbow tendinopathy with studies reporting no complications, decreased pain (>60% reduction) and/or high patient satisfaction (70% - 100%).7-14 While the sample sizes for most of these studies were small with 30 participants or less,7-14 our most recent study of 131 patients undergoing 144 procedures at the elbow had a 0% complication rate, decreased pain, and 70% patient satisfaction rate.13 Larger studies examining the risk of USGT for a variety of upper and lower extremity tendinopathies are needed to inform clinical recommendations for patients and insurance policies regarding reimbursement.
The purpose of this retrospective study was to identify the types and frequency of risks associated with USGT in a large number of patients. A secondary aim of the study was to quantify potential benefits of this procedure by observing change in pain, quality of life, and patient satisfaction. To maximize the number of procedures that could be analyzed, we included data from the elbow (extensor and flexor tendons), knee (patellar tendon), midportion Achilles, insertional Achilles, and plantar fascia sites.
Methods
Informed consent was waived and approved by the human subjects review board for the retrospective review of charts dated between September 2013 and June 2017 of all patients who had USGT for elbow tendons (common flexor and common extensor), patellar tendon, Achilles tendon (midportion and insertional), and plantar fascia. Review found a total of 262 patients (N = 87 elbow; N = 38 patellar; N = 23 midportion Achilles; N = 34 insertional Achilles; N = 80 plantar fascia). Some patients had more than one procedure performed during the study period making for a total of 289 procedures.
Data on complications were included from contact with the patient at any time point between date of procedure and long-term follow-up. Complication screening included infection, tendon rupture, hypersensitivity, or other as reported by the patient. Outcomes assessing pain and quality of life were assessed at baseline prior to the procedure, short-term follow up (6 weeks or 12 weeks), and long-term follow up (median 1.7 to 3.6 years depending on location). Short-term follow up data was gathered in clinic, while long-term follow up data was collected through online survey or phone survey which was also approved by the human subjects review board. Pilot testing for long-term follow-up was performed with the insertional AT group.6 Based on our high survey completion rate and lack of information about quality of life, the SF-12 was added for the other sites to the longterm follow-up assessment.
Pain assessments varied by tendinopathy type depending on region-specific questionnaires. Pain was assessed for the patellar group on a 4-point scale modified from the Kujala scale: None, Slight and Occasional, Occasionally Severe, and Severe/Almost always present. Pain was assessed for the midportion Achilles, insertional Achilles, and plantar fascia groups on a 4-point scale from the American Orthopedic Foot and Ankle Score (AOFAS): None, Mild/Occasional, Moderate/Daily, and Severe/Constant. For the elbow tendon group (common flexor tendon and common extensor tendon), pain was assessed on a 4-point scale adopted from the Mayo Performance Scale: None, Mild/Occasional, Moderate/ Daily, and Severe/Constant.
For all patients, quality of life was assessed using the Physical Component Summary (PCS) and Mental Component Summary (MCS) of the Short-Form 12-Item Survey (SF-12).15 The SF-12 compares the study sample to the general population with t scores (mean 50; standard deviation 10).
Patient satisfaction at short term follow up was on a 5-point scale from Very satisfied (1) to Very dissatisfied (5). Patients were asked about any procedure-related complications at routine follow-up clinic visits (2-weeks, 6-weeks, 12-weeks). In addition, participants were asked to report any procedure-related complications at longterm follow-up via phone/email.
Procedure Description
General
All patients underwent a thorough clinical evaluation including a diagnostic ultrasound. Those with chronic clinical symptoms (> 3 months) and ultrasound findings amendable to treatment with USGT (regions of degenerative or calcified tissue) were indicated for the procedure. Mean duration of symptoms prior to procedure was calculated based on number of patients who responded: 15 months for patellar, 38.9 months for non-insertional Achilles, 26 months for insertional Achilles, 31 months for plantar fascia, and 22 months for elbow. Other treatment options were discussed with patients including physical therapy, injections, extracorporeal shockwave therapy, and traditional surgical approaches. However, most patients had failed multiple prior treatments including formal physical therapy (elbow: 49, patellar: 12, midportion Achilles: 17, insertional Achilles: 22, plantar fascia: 40) and cortisone injections (elbow: 47, patellar: 2, midportion Achilles: 2, insertional Achilles: 5, plantar fascia: 41).
All procedures were performed by the senior author (MMH) who is a sports medicine physician with fellowship training in diagnostic musculoskeletal and sports ultrasound and ultrasound guided procedures. The procedures were performed in an outpatient clinical procedure suite using sterile technique including sterile ultrasound transducer covers and sterile acoustic coupling gel. Live continuous ultrasound guidance was used throughout the procedures with either an iU22 or EPIQ ultrasound cart (Philips Healthcare, Bothell, WA) and a high frequency linear transducer (12-5 or 18-5 MHz). Anesthesia was achieved with local infiltration of either 1% lidocaine without epinephrine or a 50:50 mixture of 1% lidocaine without epinephrine and 0.5% ropivacaine. Between 5 and 10 mL of local anesthetic was used based on location and patient comfort. No one required sedation. A #11 blade was used to make an approximately 5 mm skin incision and create a tract to the tendon/fascia. This was always performed in-line with the tendon/fascia to avoid iatrogenic horizontal laceration of the fibers. The TX 1 or TX 2 device (Tenex Health, Lake Forest, CA) was then used to perform all tenotomy and debridement procedures. The TX 1 and TX 2 devices operate in an identical fashion utilizing ultrasound energy to cut and debride tissue while allowing for local saline irrigation and aspiration of the debrided tissue. Differences in the two devices include only length and external fabrication (plastic vs metal sheath). All procedures aimed to debride regions of degenerative or calcific tissue with special considerations at each location as detailed below.
The post-procedure protocol was individualized based on location and extent of pathology and desired functional demands. However, our general approach was 2 weeks of rest and then progressive rehabilitation. Pain free active range of motion was started on post-procedure day 1 for all procedure sites. Following treatment of the plantar fascia or Achilles tendon, patients could weight bear as tolerated, but a protective walking boot was used for the first 1-2 weeks. Partial weight bearing on crutches or full weight bearing in a knee immobilizer was prescribed for 1-2 weeks following patellar tendon debridement. All elbow patients had a 5-pound lifting restriction for the first 6 weeks. The soonest anyone could return to full activity was 6 weeks; however, most patients returning to sports activity or manual labor required 12 weeks prior to full clearance. Near the end of our study period a criterion-based rehabilitation protocol was adopted based on our experience (Tables 1,2).
Table 1.
Criterion Based Rehabilitation Progression Following Ultrasound Guided Tendon Debridement of the Upper Limb
| Rehabilitation Phase | Estimated Timeline† | Special considerations | Restrictions | Goals | Functional test to progress to next phase |
|---|---|---|---|---|---|
| 1 | 0-2 weeks | Early ROM encouraged starting day after procedure |
|
|
|
| 2 | 2-6 weeks | Pain < 3/10 with all activities |
|
|
|
| 3 | 6+ weeks | N/A |
|
|
|
| 4 | 12+ weeks | Not applicable for all patients | N/A |
|
|
NWB, Non-weight-bearing; ROM, Range of motion; PWB, Partial weight-bearing; WBAT, Weight-bearing as tolerated; RTP Return to play; AT, Athletic trainer; S&C, Strength and conditioning; HEP, Home exercise program
† To be used as a general guide based on biologic tissue healing. This timeline does not consider the location and extent of diseased tissue as well as other intrinsic patient factors that may impact time to clinical healing.
‡ Basic load progression principles: Pain level <3/10 with activity. Any pain associated with the activity should not persist into the following day. If pain persists then load needs to be decreased.
Table 2.
Criterion Based Rehabilitation Progression Following Ultrasound Guided Tendon Debridement of the Lower Limb
| Rehabilitation Phase | Estimated Timeline† | Special considerations | Restrictions | Goals | Functional test to progress to next phase |
|---|---|---|---|---|---|
| 1 | 0-2 weeks | Early NWB pain free ROM encouraged starting day after procedure |
|
|
|
| 2 | 2-6 weeks | Pain < 3/10 with all activities |
|
|
|
| 3 | 6+ weeks | N/A |
|
|
|
| 4 | 12+ weeks | Not applicable for all patients | N/A |
|
|
NWB, Non-weight-bearing; ROM, Range of motion; PWB, Partial weight-bearing; WBAT, Weight-bearing as tolerated; RTP Return to play; AT, Athletic trainer; S&C, Strength and conditioning; HEP, Home exercise program
† To be used as a general guide based on biologic tissue healing. This timeline does not consider the location and extent of diseased tissue as well as other intrinsic patient factors that may impact time to clinical healing.
‡ Basic load progression principles: Pain level <3/10 with activity. Any pain associated with the activity should not persist into the following day. If pain persists then load needs to be decreased.
Elbow
A detailed description of our technique at the elbow has been previously reported.13 When treating the common extensor tendon, the patient was placed in a supine position with the head of the table raised approximately 30 degrees. The elbow was in slight flexion with the forearm pronated and resting on the table. A distal to proximal approach was used. Care was taken to avoid the radial collateral ligament during both the incision and the debridement with the TX device as to not inadvertently destabilize the elbow.
The common flexor/pronator tendon was approached with the patient in a supine or side lying position with the elbow in extension and forearm supinated. The location of the ulnar nerve was confirmed and carefully monitored throughout the procedure with ultrasound. Keeping the elbow in relative extension provided additional protection to the nerve in cases of subtle instability. A similar distal to proximal approach was used.
Patellar Tendon
Patients were in a supine position with the knee flexed to approximately 30 degrees and supported on a pillow. Two separate techniques were used depending on the location and extent of pathology. A more standard distal to proximal approach allowed for debridement of the patellar attachment but required division of the superficial tendon fibers for introduction of the TX device (Figure 1). When the region of pathology predominately involved the deep fibers with posteriorly projecting nodularity into Hoffa’s fat pad, a lateral to medial approach was taken (Figure 2). This allowed for more complete debridement of the involved tendon and limited debridement of the fat pad which typically becomes adherent to the region of tendinosis in these cases. The superficial fibers were completely spared with this second technique. Care was taken to avoid injury to the healthy portion of the tendon during the incision given the change to a short axis image of the tendon. The scalpel blade was maintained in an orientation longitudinal to the tendon fibers to further avoid potential for horizontal laceration.
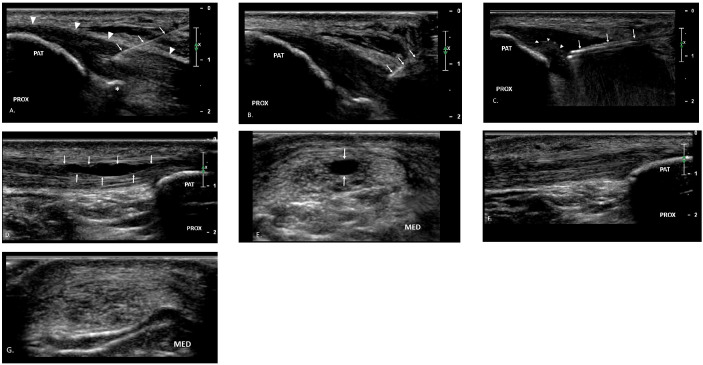
Figure 1. Distal to proximal approach to patellar tendon.
A. A 25 g 50 mm needle (arrows) is used to infiltrated local anesthetic into the subcutaneous tissues and into the patellar tendon (arrowheads). A small hyperechoic calcification is appreciated at the deep aspect of the tendon (asterisks). B. #11 blade (arrows) creates a tract into the tendon. C. The TX device (arrows) is guided into the region of pathologic tissue and debridement is performed. Hyperechoic micro-bubbles can be seen at the site of debridement (arrowheads) confirming the tissue has been removed. A companion case demonstrates long axis (D) and short axis (E) pre-procedure imaging with a partial thickness intrasubstance tear (arrows). Follow up imaging at 12 weeks post-procedure shows healing of tendon fibers in both long axis (F) and short axis (G). Patient was pain free and had returned to competitive running without limitation. PAT = patella, PROX = proximal, MED = medial.
Figure 2. Lateral to medial approach to patellar tendon.

A. Short axis image of the patellar tendon (arrowheads) showing the TX device (arrows) advanced through the lateral tract created by the #11 blade and positioned just deep/posterior to the lateral patellar tendon fibers. The device is then used to debride the adherent fatty tissue as it is advanced into the region of degenerative tendon (+) which is then debrided. B. Long axis image of the patellar tendon (arrowheads) shows an out of plane view of the TX device microtip positioned within the hypoechoic degenerative tissue (+). Using both in plane and out of plane imaging relative to the device throughout the procedure is helpful to determine adequate treatment of the entire pathologic region of tendon while also avoiding the healthy superficial fibers. PAT = patella, PROX = proximal, MED = medial.
Achilles Tendon
Patients were positioned prone with feet hanging free off the edge of the table to allow access to either the midportion or insertion of the Achilles tendon. We have previously published our technique for debridement of the Achilles insertion.6 Midportion pathology was addressed in a similar manner with a distal to proximal approach with both scalpel and device introduced in the longitudinal axis of the tendon. The location of the sural nerve was confirmed prior to incision in all cases.
Plantar Fascia
Patient positioning was identical to the Achilles. Pre-procedure ultrasound imaging identified the medial calcaneal sensory nerve and lateral plantar nerve prior to incision. A medial approach was taken to the central cord origin. If there was concomitant lateral cord origin involvement, the TX device was advanced to this location from the medial side. In only rare cases of isolated lateral cord involvement was a lateral approach taken. If a lateral approach was considered, the location of the sural nerve was confirmed during the pre-procedural ultrasound imaging.
Analysis
Descriptive statistics were used to report patient satisfaction and complications (type, frequency) by site. In order to minimize sample bias and maximize the inclusion of all patients identified in the retrospective review, data on patient satisfaction and complication rate were included from any time point. Descriptive statistics were also used to report sample characteristics by group (tendinopathy/fasciopathy site). Non-parametric and parametric tests (Wilcoxon Signed Rank test for pain as a categorical variable; Paired t-test for quality of life as a continuous variable) were used to compare outcomes at baseline (prior to USGT) to follow-up (short-term or long-term). Only a single side per patient was used for analysis in the 27 patients who had USGT on both sides to fulfill the assumption of independence of observations. Due to high rates of missing data, we chose not to impute data for missing values in the statistical analysis. Patient reported outcomes in tables reflect all available responses at each time point and analyses of change reflect all available pairs for comparison between time points. Statistical significance was defined by p ≤ 0.05.
Results
The sample was mostly adults aged 40 to 60 years, except for the relatively younger patellar tendinopathy group. The mean age ± standard deviation (SD) was 48.8 ± 9.0 years for the medial/lateral elbow group (N=87), 27.1 ± 12.9 years for the patellar group (N=38), 50.6 ± 15.4 for the midportion Achilles group (N=23), 52.2 ± 11.6 years for the insertional Achilles group (N = 34), and 47.2 ± 12.2 for the plantar fascia group (N=80). The average body mass index (BMI) was in the obese category, except for the patellar tendon group which was in the overweight category. The mean BMI ± SD was 31.3 ± 10.0 kg/m2 for the medial/lateral elbow group, 26.2 ± 7.6 kg/m2 for the patellar group, 30.0 ± 7.8 kg/ m2 for the midportion Achilles group, 32.9 ± 7.5 kg/m2 for the insertional Achilles group, and 30.4 ± 6.4 kg/m2 for the plantar fascia group. The percentage of women varied by group (Elbow, 46%; Patellar, 24%; midportion AT, 65%; insertional AT 62%; plantar fascia, 76%).
There was a low complication rate of 0.7% (2/289 cases), including 1 superficial wound infection that resolved with oral antibiotics and 1 case of wound hypersensitivity. No serious complications were reported. Prior to USGT (baseline) the majority of patients reported, by region specific questionnaire, moderate/daily pain that decreased by short-term (2 to 12 weeks) and long-term (median 1.7 to 3.6 years depending on location) follow-up to mild/occasional pain (Table 3). In parallel, prior to USGT most patients reported abnormally low physical function (≥1 SD below the general population mean t-score of 50 on SF-12, PCS) yet by long-term follow-up patients were within normal range of physical function (Table 4). In contrast, at baseline our sample reported above average mental health scores, and therefore any improvement in MCS was minimal for all groups (Table 5). Additionally, patient satisfaction at short-term follow up (2, 6 or 12 weeks) was reported for each group with a majority reporting either ‘very satisfied’ or ‘somewhat satisfied’ (Table 6).
Table 3.
Pain Measured as: 0, No Pain; 1, Mild/Occasional; 2, Moderate/Daily; 3, Severe/Constant Data Presented as Median [Interquartile Range]. Analyses Used Pair-Wise Deletion for Missing Data
| Elbow | Patellar | Achilles - Midportion | Achilles – Insertional | Plantar Fascia | |
|---|---|---|---|---|---|
| Baseline | 2.0 [2.0 to 2.0] | 2.0 [1.0 to 2.0] | 2.0 [2.0 to 2.5] | 2.0 [2.0 to 2.0] | 2.0 [2.0 to 3.0] |
| Short-term | 1.0 [1.0 to 1.5]* | 1.0 [1.0 to 1.0] | 1.0 [0.0 to 2.0] | 1.0 [1.0 to 2.0]* | 1.0 [1.0 to 2.0]* |
| Number analysis, of p-value pairs for | n=34, p < 0.01 | NA | n=5, NA | n=23, p < 0.01 | n=39, p < 0.01 |
| Long-term | 0.0 [ 0.0 to 1.0]* | 1.0 [0.0 to 1.0]* | 1.0 [0.0 to 1.0]* | 1.0 [1.0 to 1.0]* | 1.0 [0.0 to 1.0]* |
| Number of pairs for analysis, p-value | n=37, p < 0.01 | n=16, p = 0.03 | n=13, p < 0.01 | n=17, p <0.01 | n=42, p < 0.01 |
NA, Not applicable due to paired samples n<10.
Sample size for descriptive statistics varies by group and time point: Elbow survey responders (Baseline, short term, long term): n = 57, 53, 59. Patellar survey responders (Baseline, short term, long term): n = 32, 2, 20. Midportion Achilles survey responders (Baseline, short term, long term): n = 17, 7, 17. Insertional Achilles total sample: n = (Baseline, short term, long term): n = 27, 26, 20. Plantar fascia (Baseline, short term, long term): n = 63, 52, 53.
Significant p-values are bolded.
*Significant differences relative to baseline, Wilcoxon signed rank tests, p < 0.05
Table 4.
Function Measured with the SF-12, Physical Component Summary (PCS) and Presented as Mean ± SD The General Population Mean t-score= 50 and SD=10 Analyses Used Pair-Wise Deletion for Missing Data
| Elbow | Patellar | Achilles - Midportion | Achilles – Insertional | Plantar Fascia | |
|---|---|---|---|---|---|
| Baseline | 36.7± 6.5 | 41.7 ± 9.4 | 36.8± 9.8 | 40.8±9.4 | 36.0± 9.4 |
| Short-term | 41.7± 9.1* | 33.6 ± 1.3 | 42.7 ± 10.2 | 44.0±7.1* | 40.1± 9.4* |
| p-value | p < 0.01 | NA | NA | p = 0.03 | p < 0.01 |
| Long-term | 48.0± 5.5* | 49.2 ± 3.9 | 49.2± 3.7* | Not Reported | 48.0± 6.1* |
| p-value | p < 0.01 | p = 0.20 | p < 0.01 | N/A | p < 0.01 |
NA, Not applicable due to paired samples n<10.
Sample size for descriptive statistics varies by group and time point: Elbow (Baseline, short term, long term): n = 71, 63, 58. Patellar (Baseline, short term, long term): n = 28, 2, 20. Midportion Achilles (Baseline, short term, long term): n = 17, 6, 17. Insertional Achilles (Baseline, short term, long term): n = 24, 23, N/A. Plantar fascia (Baseline, short term, long term): n = 59, 51, 54. Significant p-values are bolded.
*Significant differences relative to baseline, paired t-test, p < 0.05
Table 5.
Mental Component Score (MCS) of the SF-12 Presented as Mean ± SD The General Population Mean T-Score= 50 and SD=10 Analyses Used Pair-Wise Deletion for Missing Data
| Elbow | Patellar | Achilles - Midportion | Achilles – Insertional | Plantar Fascia | |
|---|---|---|---|---|---|
| Baseline | 56.4± 9.4 | 59.4 ± 4.8 | 52.6± 11.5 | 59.4±5.2 | 57.5± 8.3 |
| Short-term | 57.2± 8.4 | 63.5 ± 0.5 | 62.2± 4.4 | 59.8±3.7 | 57.9± 8.7 |
| p-value | p = 0.51 | NA | NA | p > 0.05 | p = 0.62 |
| Long-term | 54.1± 8.7 | 57.7 ± 2.5 | 55.5± 5.0 | Not Collected | 56.8± 5.2 |
| p-value | p = 0.07 | p = 0.15 | p = 0.56 | N/A | p = 0.24 |
NA, Not applicable due to paired samples n<10.
Sample size for descriptive statistics varies by group and time point: Elbow (Baseline, short term, long term): n = 71, 63, 58. Patellar (Baseline, short term, long term): n = 28, 2, 20. Midportion Achilles (Baseline, short term, long term): n = 17, 6, 17. Insertional Achilles (Baseline, short term, long term): n = 24, 23, N/A. Plantar fascia (Baseline, short term, long term): n = 59, 51, 54. Significant p-values are bolded.
*Significant differences relative to baseline, paired t-test, p <0.05
Table 6.
Patient Satisfaction at Short-Term Follow-up: 2 weeks (Patellar, Midportion Achilles); 6 or 12 Weeks (Elbow, Insertional Achilles, Plantar Fascia) Values Presented as Number (% sample / % of respondents at short-term follow-up)
| Elbow | Patellar | Achilles - Midportion | Achilles – Insertional | Plantar Fascia | |
|---|---|---|---|---|---|
| Very Satisfied | 23 (24%/38%) | 14 (34%/54%) | 11 (42%/92%) | 14 (41%/45%) | 25(31%/44%) |
| Somewhat Satisfied | 20 (21%/33%) | 7 (17%/27%) | 0 (0%/0%) | 10 (29%/32%) | 11(14%/19%) |
| Neutral | 7 (7%/12%) | 5 (12%/19%) | 1 (4%/8%) | 5 (15%/16%) | 9(11%/16%) |
| Somewhat Dissatisfied | 6 (6%/10%) | 0 (0%/0%) | 0 (0%/0%) | 2 (6%/7%) | 7(9%/12%) |
| Very Dissatisfied | 4 (4%/7%) | 0 (0%/0%) | 0 (0%/0%) | 0 (0%/0%) | 5(6%/9%) |
| Missing | 27 (37%/NA) | 15 (37%/NA) | 14 (54%/NA) | 3 (9%/NA) | 30(38%/NA) |
Elbow survey responders: n = 60; Patellar survey responders: n = 26; Midportion Achilles survey responders: n = 12; Insertional Achilles survey responders: n = 31; Plantar fascia survey responders: n = 57.
Discussion
This is currently the largest known study to date examining the safety and effectiveness of USGT with 262 patients and 289 procedures. The main study finding indicates USGT is a safe procedure with secondary findings indicating reduced pain, improved function, and high patient satisfaction over time.
There was a complication rate of 0.7%, which supports this procedure being considered low risk. This contrasts with reports of multiple complications in two recent case series of patients with Achilles tendinopathy treated with USGT using the TX device.3,4 Sanchez et al. reported on six patients with various complications including worsening pain, partial tearing, and DVT and Gurin et al. reported two midportion ruptures following USGT.3-4 However, based on their description of the cases, inappropriate technique or post-surgical rehabilitation was likely the primary contributor to the poor outcomes. They reported transverse tears which they speculated were the result of the ultrasound guided tenotomy. We recommend a longitudinal approach as described above with both the incision and debridement to avoid such transverse fiber disruption. Also, multiple cases report prolonged immobilization in a walking boot post-procedure which is not in line with current best practice. Tendons require loading to facilitate healing and early introduction of appropriate loading is one of the primary advantages to the minimally invasive technique. However, while early load introduction is important, progression must be in line with expected tendon healing. Details regarding appropriate load progression in the cases of tendon rupture were not available and may have contributed to the poor outcomes. While it is important to identify complications and risks of all procedures, it is also important to carefully assess contributors to those risks.
By 6 to 12 week follow up, reported pain decreased from moderate/daily pain to mild/occasional pain. The greatest pain reduction occurred in the elbow group, where pain further improved by long-term follow-up with a median pain rating of “None.” This reduction in pain was consistent with multiple other studies that reported decreased post- procedure pain measured by the visual analog scale of pain (VAS).7,9,12,14 Seng et al. reported a decrease in pain from 0.5 ± 0.70 at 12 months to 0 ± 0.9 out of 10 at 36 months.12 Barnes et al. and Boden et al. also noted similar improvements in VAS pain scores.7,9
At baseline, all groups had impaired physical function (SF-12 PCS 36.0 to 41.7). By short term follow up, most groups had small yet, statistically significant improvement in function (SF-12 PCS 40.1 to 44.0). Due to small sample size at short-term follow-up, the change from baseline to short-term was not statistically compared for the patellar tendon (n=2 at 6 weeks) and midportion Achilles groups (n=4 at 6 weeks, n=2 at 12 weeks). By long-term follow up, all groups reported physical function at a similar level as the general population mean (SF-12 PCS 48.0 to 49.2). A limitation of this study is lack of region-specific outcome measures, yet this global improvement in function is consistent with Boden et al. who reported improvements in function measured by the Quick Dash. Scores improved from 35.9 ± 5 to 12.5 ± 3.4 (p<0.01).9 Significant improvement in the MCS component of the SF-12 survey was not found in any group. It should also be noted that the MCS was not impaired at baseline for any group and no further increase in this component was seen. Together, this indicates that USGT may affect quality of life related to physical function rather than quality of life related to the mental component.
Patient satisfaction at 6 or 12 weeks was collected in the elbow, insertional Achilles, and plantar fascia groups. Due to low response rate at 6- and 12-week follow-up for the patellar and midportion Achilles groups, patient satisfaction at 2 weeks was also included. The majority of respondents in the elbow, insertional Achilles, and plantar fascia groups (71%, 70%, and 63% respectively) reported either ‘very satisfied’ (or ‘somewhat satisfied’ at 6 or 12 weeks. Similarly, the majority of respondents in the patellar tendon and midportion Achilles groups (81% and 92% respectively) reported either ‘very satisfied’ or ‘somewhat satisfied’ at 2 weeks (Table 4). Despite patient reported improvements in pain or physical function a year post procedure, patients were reporting satisfaction as soon as 2 weeks post procedure. This was consistent with multiple other studies reporting high rates of patient satisfaction (≥ 70%).5,7,8,10-14
The generalization of these findings to all patients considering USGT is limited due the potential bias of missing data at each time point. Long term response rate was 63%; however, response rate at either short or long term was 95% allowing for a higher capture rate of any complication. Still, due to limits in response rate, this data should be interpreted with caution until larger prospective studies with region-specific outcome measures can better determine the safety and effectiveness of USGT. The analysis was adjusted accordingly with statistical comparisons over time limited to only those patients with baseline data. Therefore, patients with only short-term and/or long-term follow-up but not baseline data are included in descriptive data in tables, but not in statistical comparison over time. Patients without baseline data did not appear to differ from the overall sample, and descriptive data on all available patients was included to minimize selective sampling.
Finally, USGT has many potential benefits compared to traditional surgical management of chronic tendinopathy. The procedure is safely performed in an outpatient setting under local anesthesia. Not only does this limit anesthesia associated risks, but also significantly reduces cost. At our institution, an open surgical tenotomy costs approximately 3 times that of an USGT with real cost savings of up to $18,000 as we have previously reported.6 The minimally invasive nature provides several benefits. The reduction in post-procedure pain obviates the need for opioid pain medication and therefore reduces risk of potential opioid dependence. As healthy tissues are relatively spared, there is an accelerated rehabilitation protocol with faster return to work and sport related activities. Overall, this study demonstrates that with appropriate technique and post-procedural management USGT is a safe procedure.
Conclusion
To date, this is the largest study exploring the safety of USGT with a total of 262 patients and 289 procedures. This procedure was found to be safe as there was a low complication rate of 0.7%. Patients also experienced reduced pain and improved physical function at short term and long term follow up. This study supports future research of USGT including prospective studies comparing USGT to the accepted standard of care.
Acknowledgments
Study data was collected and managed using REDCap electronic data capture tools hosted at University of Iowa (supported by NIH 54TR001013). Two subsets of this data have been previously published by the same authors (Insertional Achilles, N = 34; elbow, N = 131) and is reproduced with permission as discussed in the text. A preprint of this manuscript can be found at https://www.researchsquare.com/article/rs-32408/v1.
Availability of Data and Materials
Individual participant data that underlie the results reported in this article after deidentification will be available to immediately following publication and ending 5 years following publication. This data will be shared with researchers who provide a methodologically sound proposal and will use the data to achieve aims specified in the proposal. Proposals should be directed to Mederic-hall@uiowa.edu. To gain access, data requestors will need to sign a data access agreement.
References
- 1.Scott A, Squier K, Alfredson H, et al. ICON 2019: International Scientific Tendinopathy Symposium Consensus: Clinical Terminology. Br J Sports Med. 2019. [DOI] [PubMed]
- 2.Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37(9):1855–1867. doi: 10.1177/0363546508324283. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez PJ, Grady JF, Saxena A. Percutaneous Ultrasonic Tenotomy for Achilles Tendinopathy Is a Surgical Procedure With Similar Complications. J Foot Ankle Surg. 2017;56(5):982–984. doi: 10.1053/j.jfas.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Gurin D, Sultan AA, Berkowitz M, Miniaci-Cox-head SL. Spontaneous Closed Rupture of Achilles Tendon Following Minimally Invasive Ultrasonic Energy Therapy: A Report of Two Cases. Surg Technol Int. 2019;34:483–487. [PubMed] [Google Scholar]
- 5.Freed L, Ellis MB, Johnson K, Haddon TB. Fasciotomy and Surgical Tenotomy for Chronic Achilles Insertional Tendinopathy A Retrospective Study Using Ultrasound-Guided Percutaneous Microresection. J Am Podiatr Med Assoc. 2019;109(1):1–8. doi: 10.7547/15-168. [DOI] [PubMed] [Google Scholar]
- 6.Chimenti RL, Stover DW, Fick BS, Hall MM. Percutaneous Ultrasonic Tenotomy Reduces Insertional Achilles Tendinopathy Pain With High Patient Satisfaction and a Low Complication Rate. J Ultrasound Med. 2019;38(6):1629–1635. doi: 10.1002/jum.14835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes DE, Beckley JM, Smith J. Percutaneous ultrasonic tenotomy for chronic elbow tendinosis: a prospective study. J Shoulder Elbow Surg. 2015;24(1):67–73. doi: 10.1016/j.jse.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Battista CT, Dorweiler MA, Fisher ML, Morrey BF, Noyes MP. Ultrasonic Percutaneous Tenotomy of Common Extensor Tendons for Recalcitrant Lateral Epicondylitis. Tech Hand Up Extrem Surg. 2018;22(1):15–18. doi: 10.1097/BTH.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 9.Boden AL, Scott MT, Dalwadi PP, Mautner K, Mason RA, Gottschalk MB. Platelet-rich plasma versus Tenex in the treatment of medial and lateral epicondylitis. J Shoulder Elbow Surg. 2019;28(1):112–119. doi: 10.1016/j.jse.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Hall MM, Woodroffe L. Ultrasonic Percutaneous Tenotomy for Recalcitrant Calcific Triceps Tendinosis in a Competitive Strongman: A Case Report. Curr Sports Med Rep. 2017;16(3):150–152. doi: 10.1249/JSR.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 11.Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: Early clinical experience with a novel device for minimally invasive percutaneous microre-section. Am J Sports Med. 2013;41(3):636–644. doi: 10.1177/0363546512470625. [DOI] [PubMed] [Google Scholar]
- 12.Seng C, Mohan PC, Koh SB, et al. Ultrasonic Percutaneous Tenotomy for Recalcitrant Lateral Elbow Tendinopathy: Sustainability and Sonographic Progression at 3 Years. Am J Sports Med. 2016;44(2):504–510. doi: 10.1177/0363546515612758. [DOI] [PubMed] [Google Scholar]
- 13.Stover D, Fick B, Chimenti RL, Hall MM. Ultrasound-guided tenotomy improves physical function and decreases pain for tendinopathies of the elbow: A retrospective review. J Shoulder Elbow Surg. 2019. [DOI] [PMC free article] [PubMed]
- 14.Williams RC, Pourcho AM. Percutaneous Ultrasonic Tenotomy for Refractory Common Extensor Tendinopathy After Failed Open Surgical Release: A Report of Two Cases. PM R. 2018;10(3):313–316. doi: 10.1016/j.pmrj.2017.07.077. [DOI] [PubMed] [Google Scholar]
- 15.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data that underlie the results reported in this article after deidentification will be available to immediately following publication and ending 5 years following publication. This data will be shared with researchers who provide a methodologically sound proposal and will use the data to achieve aims specified in the proposal. Proposals should be directed to Mederic-hall@uiowa.edu. To gain access, data requestors will need to sign a data access agreement.