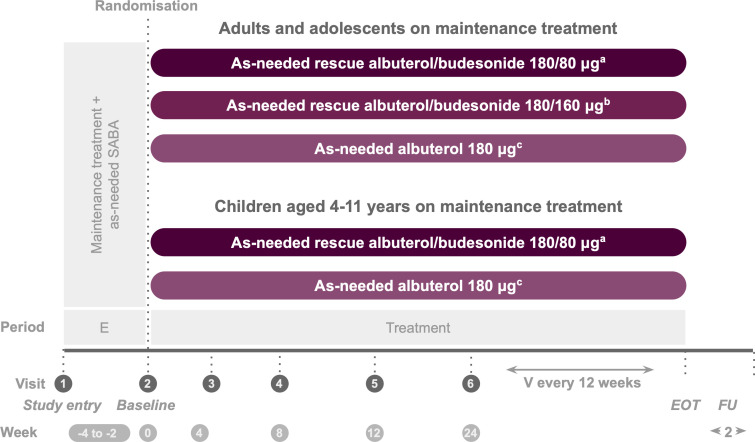
Figure 1.
Study design. aTwo inhalations albuterol/budesonide pMDI 90/40 µg. bTwo inhalations albuterol/budesonide pMDI 90/80 µg. cTwo inhalations albuterol pMDI 90 µg. The screening/enrolment period was 2–4 weeks except if a severe exacerbation occured during this time, in which case it was ≤9 weeks. During screening, patients discontinued their usual rescue medication and used as-needed albuterol sulfate 180 µg. E, enrolment; EOT, end of treatment; FU, follow-up; pMDI, pressurised metered-dose inhaler; SABA, short-acting beta2-agonists; V, visit.