Abstract
Flagellin is an immunodominant antigen in Crohn’s disease, with many patients showing anti-flagellin antibodies. To study the clonality of flagellin-reactive CD4 cells in Crohn’s patients, we used a common CD154-based enrichment method following short term antigen exposure to identify antigen-reactive CD4 cells. CD154 expression and cytokine production following antigen exposure compared to negative control responses (no antigen exposure) revealed that only a small fraction of CD154-enriched cells could be defined by antigen-reactive cytokine responses. This was especially true for low frequency flagellin-reactive CD4 cells compared to polyclonal stimulation or C. albicans antigen exposure. Moreover, we found that culture conditions used for the assay contributed to background CD40 ligand (CD154) expression in the CD154-enriched CD4 cells. Using a cut-off rule based on flow cytometry results of the negative control CD154-enriched CD4 cells, we could reliably find the fraction of antigen-reactive cells in the CD154-enriched population. Antigen-reactive CD4 cytokine production was restricted to CD4 cells with an effector memory phenotype and the highest levels of induced CD154 expression. This has important implications for identifying antigen-specific T cells of interest for single cell cloning, phenotyping and transcriptomics.
Keywords: CD4 T cells, Flagellin, Crohn’s disease, CD40 Ligand, Cytokines response
Introduction
Gut microbial antigen-specific CD4+ T cells play a central role in experimental murine colitis and are thought to mediate the chronic intestinal inflammation of Crohn’s disease [1, 2]. The gut microbial antigen flagellin has been characterized as an immunodominant antigen in Crohn’s patients [3] and an aberrant adaptive immune response to flagellin proteins is well known in Crohn’s disease [4, 5]. The diagnosis of Crohn’s is supported by the presence of serum antibodies against flagellin antigens (CBir1, A4-Fla2, Fla3, and FlaX) [6] and this serologic marker is associated with worse disease outcomes [7, 8]. Moreover, Crohn’s disease patients widely share a small number of T cell receptor CDR3 regions, some of which are associated with flagellin reactivity [9]. However, it is unknown whether these immune responses to flagellin antigens themselves primarily cause the inflammatory damage in Crohn’s disease. Gaining insight into flagellin antigen (Ag)-specific T cell-mediated disease mechanisms could provide a rationale to target antigen-specific T cells as novel therapies.
To associate highly shared Crohn’s TCR clonotypes with antigen-specificity and function, we aimed to identify flagellin-specific T cells in Crohn’s patients and characterize their cytokine profile and suitability for isolation. Several classic methods that depend on activation marker expression and cytokine production have been used to define microbe-reactive T cells including T cell proliferation assays ([3H] thymidine incorporation, CFSE dilution), cytokine secretion assays (ELISA, ELISPOT) and the so-called Activation Induced Marker (AIM) assay [10–13]. Each of these assays have their individual limitations in establishing specific details about antigen-reactive T cells since individually they may not directly determine the original frequency of Ag-reactive T cells, cannot detect whether the same cell makes multiple cytokines or measure cytokine production based on the length of observation, or may not provide the chance for isolation of single cells of interest.
Previous studies have demonstrated that CD40L (CD154) is overexpressed on circulating T cells in Crohn’s disease and the induction of CD40L is involved in pathogenic cytokine production in these patients [14, 15]. Therefore, taking advantage of this transiently expressed ligand by using the previous described CD154+ (CD40L) enrichment assay we aimed to directly enumerate and characterize antigen specific cells after short term stimulation, which lowers the risk of potential phenotypic and functional changes due to in vitro effects during prolonged culture ex vivo [16, 17].
Furthermore, antigen-reactive T cell enrichment technology [16] allows for accurate and sensitive assessment of CD4 cells and, relevant for our studies, does not require prior knowledge of the peptide-MHC complex [9–11]. Cognate antigen activated CD4 cells induce CD40L (CD154) surface expression which is important for activation of APCs and further CD4 T cell priming. Previous studies have shown that CD154-expression can be detected effectively following 7-h stimulation of healthy donor peripheral mononuclear cells (PBMC) with polyclonal stimulation (SEB, PMA/I), and fungal (C. albicans, A. fumigatus), viral (CMV, HIV Gag) and bacterial (E coli, mycobacterium) antigens [13, 16, 20]. We now report the use of Crohn’s disease-associated flagellin antigen-driven CD154 expression together with intracellular cytokine production to identify small subsets of CD4 cells which we take to be authentic antigen-reactive cells. These flagellin-reactive CD4 cells represent a plausible percentage of total circulating CD4 cells, are increased in patients with active Crohn’s disease, and can be differentiated from the increased background expression of CD154 induced by the culture conditions themselves.
Materials and Methods
Patient Donors:
For our study we recruited patients from the Birmingham VA Medical Center and the University of Alabama at Birmingham (UAB) outpatient clinics. Eligible patients had verified IBD diagnoses according to conventional clinical, endoscopic and histologic criteria [20]; current disease status was documented by clinical evaluation and endoscopic and/or imaging studies. Blood samples were obtained from Crohn’s disease (n = 38) and non-IBD healthy control patients (n = 5). Demographic and clinical characteristics are summarized in Table I. This study was approved by the IRBs of the Birmingham VA Medical Center and the University of Alabama at Birmingham. All donors involved in the study signed an informed consent before their inclusion.
Table I.
Demographics and Clinical characteristics of studied individuals
| Characteristic | All patients (n=43) | Crohn’s disease (n=38) | Non-IBD HC (n=5) |
|---|---|---|---|
| Male/Female | 25/17 | 23/15 | 3/2 |
| Median (Range) Age | 31 (19–76) | 30 (19–76) | 31 (26–71) |
|
| |||
| Concomitant Medications | |||
|
| |||
| 5-Aminosalicylates | 3 | ||
| Infliximab/adalimumab | 20 | ||
| Vedoliz/Ustekn/Certolizumab | 8 | ||
| Corticosteroids | 2 | ||
| Azathioprine/6-meracaptopurine | 9 | ||
| None | 6 | 5 | |
Lymphocyte Isolation from Human Blood:
Blood samples were collected in heparin-coated tubes and processed within 2 hours of procurement. The mononuclear cell fraction was isolated using a density gradient. Blood was diluted 1:1 with PEB [1 X Phosphate-buffered saline (PBS; Gibco), 0.5 % human serum type AB (Corning™), 2 mM EDTA (Invitrogen)] and layered on top of Ficoll-Paque Plus (GE Healthcare) and centrifuged for 25 minutes at room temperature at 400 x g with acceleration and no brake. The mononuclear cell fraction was aspirated and washed with PEB and centrifuged for 20 minutes at room temperature at 300 x g. The cell pellet was then resuspended in PEB and the number of viable cells was counted using Trypan blue (Sigma-Aldrich). The cells were then resuspended in RPMI 1640 medium with L-glutamine (Gibco, Hyclone), supplemented with 5% (v/v) AB serum (Corning™) and 25mM HEPES (Gibco) and 5 X 106 PBMCs/well were plated in 24 well plates for overnight incubation at 37°C and 5% CO2.
Antigen Stimulation and Enrichment of Peripheral mononuclear Cells:
Following an overnight incubation, the PBMCs were either left unstimulated or stimulated for 7 h with the following Ags: Candida albicans (C. albicans)-lysate (20 μg/ml; Greer Laboratories), CytoStim (1mg/ml; Miltenyi Biotec) and flagellin antigens -CBir1, -FlaX, -Fla2 (10ng/ml) which were kindly provided by Dr. C. O. Elson (University of Alabama, Birmingham, AL) in the presence of 1 mg/ml anti-CD40, 1 mg/ml anti-CD28-functional grade pure monoclonal antibody (both Miltenyi Biotec) and 1 mg/ml Brefeldin A (BD Biosciences) which was added for the last 3 h of incubation. For major histocompatibility complex-II blocking, anti–HLA-DR, -DP, -DQ (Tü39; BD Pharmingen) was added at 10 ug/mL to the culture 30 minutes before antigens and co-stimulatory molecules. Cells were kept at 37°C and 5% CO2 for the duration of the stimulation.
After stimulation, cells were separated using the CD154 MicroBead Kit (Miltenyi Biotec). The cells were indirectly magnetically labeled with CD154-biotin and antibiotin Microbeads and enriched by using MS MACS columns (Miltenyi Biotec). The surface staining was performed on the first column, followed by fixation, permeabilization (Inside stain kit; Miltenyi Biotec), and intracellular cytokine staining on a second column.
Antibody Staining and Flow Cytometry Analysis:
For surface staining of enriched cells we stained with different combinations of the following mAbs according to manufacturer’s protocols (clone names in parentheses): Live/Dead Fix NIR-80 (L34975; Life Technologies), CD3-FITC (HIT3a), CD14-allophycocyanin (M5E2), CD20-allophycocyanin (2H7), CD8-allophycocyanin (RPA-T8), CD69-Phycoerythrin-Cy5 (FN50), CD4-Phycoerythrin (RPT-T4), CD4-Alexa Fluor 700 (RPA-T4), (All Becton Dickinson Pharminogen), CD69-Pacific blue (FN50), CD62L-Brilliant Violet 421 (DREG-56), mouse IgG1k-Brilliant Violet 421 (MPOC-21), CD45RO-Brilliant Violet 421 (UCHL1), mouse IgG2ak-Brilliant Violet 421 (MPOC-173), CD4-PECy7 (RPA-T4) (All Biolegend), CD4-Phycoerythrin-Vio615 (REA623; Miltenyi Biotec), FcR blocking reagent (Miltenyi Biotec). After fixation and permeabilization (Inside stain kit; Miltenyi Biotec). We stained for CD154 expression (or isotype controls) alone using: CD154-Pacific Blue (24–31; Biolegend), mouse IgG1k-Pacific blue (MPOC-21; Biolegend), CD154-Phycoerythrin (REA238), mouse IgG2a-PE; REA Control(S)-PE, CD154-VioBlue (5C8), mouse IGg2a-VioBlue, CD154-FITC (REA238), or recombinant human IgG1-FITC (REA Control(S)-FITC); all Miltenyi Biotec, or in combination with different cytokines (or their isotype controls), using : TNF-a-Alexa Fluor 700 (MP6-XT22; Biolegend), Rat IgG1k (RTK207; Biolegend), TNF-a PECy7 (Mab; MAB1; BD Biosciences), mouse IgG1K-PECy7 (MPOC-2; BD Biosciences), TNF-a-PE-Vio770 (cA2); human IGg- PE-Vio770 (Miltenyi Biotec), REA Control (I)-PE-Vio770, IFN-g-PECy7 (4S.B3; eBioscience), IFN-g-Alexa Fluor 700 (B27; BD Biosciences), mouse IgG1K-Alexa Fluor 700 (MPOC-21; BD Biosciences), IL-17A-PerCP-Cyanine5.5 (N49–653; BD Biosciences), mouse IGg1k-PerCP-Cyanine5.5 (MOPC-21; BD Biosciences), IL-17A-PerCP-Cyanine5.5 (eBio64DEC17; eBioscience), mouse IgG1K-PerCP-Cyanine5.5 (P3.6.2.8.1; eBioscience). Stained cells were acquired on a LSRII Flow Cytometer (BD Biosciences) using FACSDIVA software (version 8.0; BD Biosciences) within 24 hours after staining and analyzed using FlowJo software (version 10.5.3; Treestar).
CD154 Time course:
10×106 PBMC were stimulated 0, 3, 7, 9 and 12h with no antigen or with the following: Candida albicans-lysate (20 μg/ml; Greer Laboratories), CytoStim (1mg/ml; Miltenyi Biotec), and combined flagellin antigens -CBir1, -Fla-X, -Fla-2 (10ng/ml) in the presence of 1 mg/ml CD40 and 1 mg/ml CD28 functional grade pure Ab (both Miltenyi Biotec). For intracellular staining, 1 mg/ml Brefeldin A (BD Biosciences) was added for the final 3h (3 hr time point) or 4h (later time points) of incubation. Cells were kept at 37°C and 5% CO2 for the duration of the stimulation and then enriched for CD154 expression as mentioned above.
Statistics:
The software package Graphpad Prism (version 7; GraphPad Software) was used to analyze data and to perform statistical analyses. Statistical significance of differences was assessed using nonparametric Mann-Whitney U tests and Kruskal Wallis with Dunn’s multiple comparison, and two-way ANOVA with Dunnett’s multiple comparison. *p<0.05, **p<0.01, ***p<0.001, ****p<0.001.
Results
Detection of Flagellin Specific T Cells Response by CD154 Expression
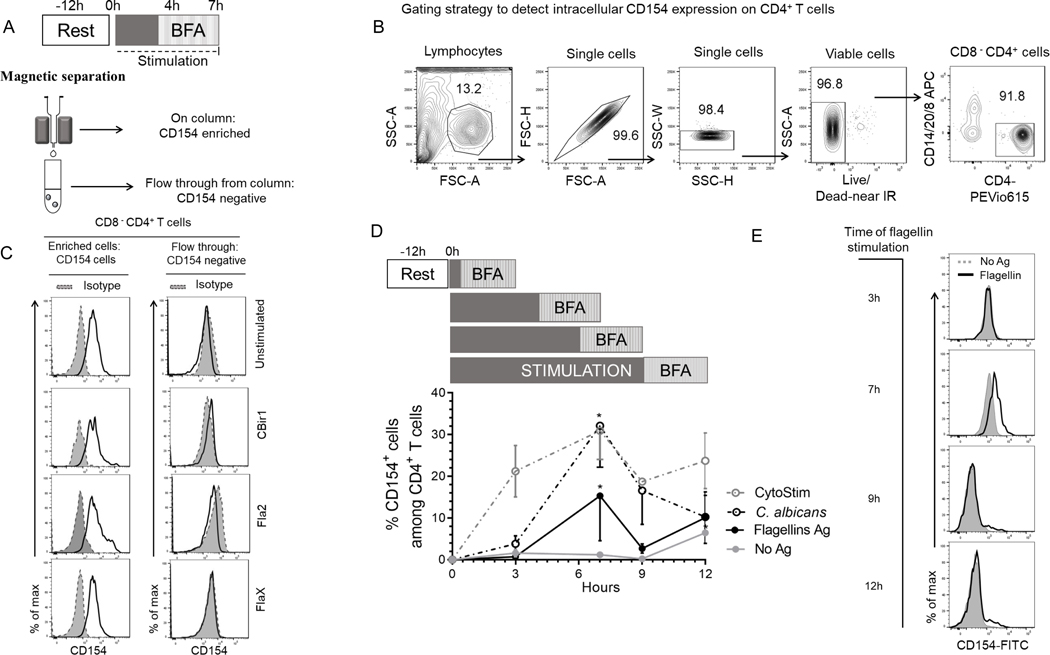
To study how CD40L (CD154) expression could be used to identify flagellin-reactive circulating CD4+ T cells from Crohn’s disease patients, we stimulated PBMCs with antigens for 7hrs and magnetically enriched for CD154 (Fig.1A). To assess CD154 expression on enriched CD154+ cells, we first gated on viable cells, then excluded non-T cell lineages (CD14+, CD20+) and CD8+ cells to obtain viable CD4+ T cells (Fig.1B). The viable CD8− CD4+ T cells were evaluated for CD154 expression. A significantly higher expression of CD154+CD4+ T cells were detected among the Ag-stimulated PBMC samples compared with unstimulated samples and CD154 isotype controls (Fig.1C and Supplemental Fig.1A). CD154 expression on the flow-through cells was similar to isotype control, showing that CD154+ cells have been efficiently retained on the column (Fig.1C). Enriched cells ranged from 400–1000 (Cytostim), 30–1000 (C. albicans), 10–100 (CBir1), 10–500 (Fla2) and 10–400 (FlaX) CD154+ cells per 105 CD4+ T cells. These results show that CD154 enrichment is sensitive enough to detect circulating flagellin-reactive CD4+ T cells from Crohn’s disease patients.
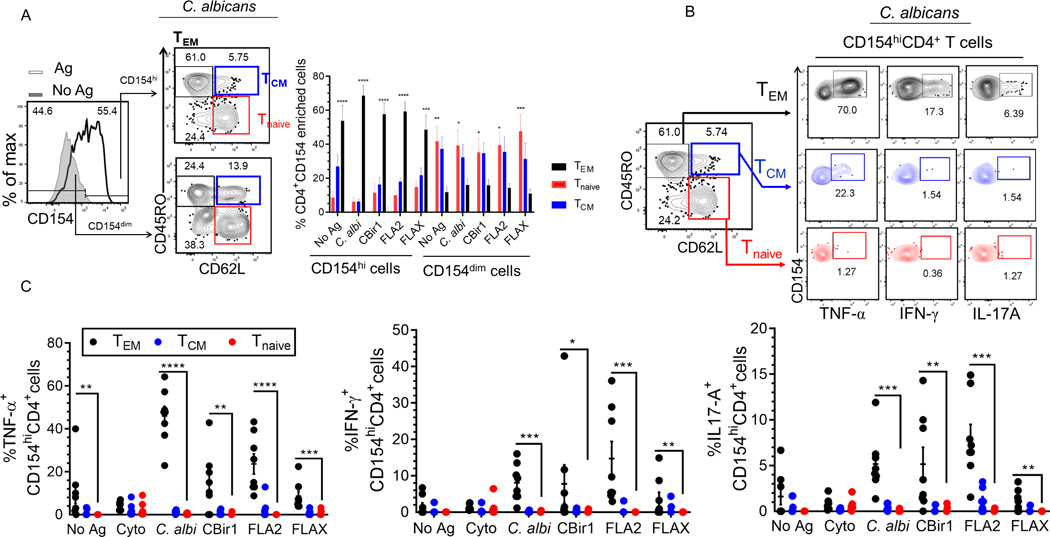
Figure 1. Detection and optimization of intracellular CD154 in CD4 T cells stimulated in the presence of BFA, anti-CD28 and anti-CD40.
(A) Experiment protocol overview. 5.0×106 PBMC from CD patients cultured with antigens and magnetically enriched for CD154 on magnetic columns. (B) Representative contour plots showing gating strategy for determining viable CD4+ T cells events. We gated on lymphocyte populations then exclude cell aggregates, dead cells, non-T cell lineages and CD8 T cells (CD14+, CD20+, CD8+). (C) Histogram representation of intracellular CD154 expression for enriched and flow through with isotype control. (D) Kinetics of intracellular CD154 in CD4+ T cells following polyclonal and antigen stimulation. Timeline of CD154 expression after stimulation periods (0, 3, 7, 9 and 12h post activation) in the presence of BFA. The percentage of viable CD154+CD4+ T cells from Crohn’s patients (n = 3) exposed to C. albicans, CytoStim and combined flagellin antigens (CBir1, Fla-2 and Fla-X), or left unexposed for 7 h. Bars represent the means ± SEM of three independent experiments. Statistical significance compared the mean for each time point for each condition using Kruskal-Wallis test with Dunn’s multiple comparison. *p<0.05. (E) Histogram representation of CD154 surface expression following stimulation with flagellin antigens, or no stimulation (No Ag).
Optimization of CD154 expression on Stimulated Crohn’s PBMCs
The optimal time for inducing CD154 expression among circulating flagellin-specific CD4+ T cells from Crohn’s disease patients is unknown. To determine the optimal duration of flagellin antigen stimulation for our assay, we evaluated the kinetics of intracellular CD154 expression. Overnight rested PBMCs were incubated with anti-CD40, anti-CD28 and brefeldin A and stimulated with combined flagellin antigens and our positive controls (C. albicans and CytoStim), or left unstimulated and the cells were removed at various time points (3h, 7h, 9h and 12h) (Fig.1D). CytoStim-stimulated CD4 T cells showed peak expression of intracellular CD154 from 3–7h, while C. albicans- and flagellin antigen-stimulated cells showed peak expression of intracellular CD154 at 7h of stimulation. Of note, we observed increased background expression of intracellular CD154 by unstimulated cells starting at the 3hr time point. Following stimulations there was a significantly higher population of CD154+ expressing cells in comparison to background (isotype control and unstimulated cells) at 7h stimulation (Figs.1D and 1E.). These data validate the use of 7h incubation times to detect intracellular CD154 expression by flagellin antigen-specific CD4+ T cells.
Identifying Cytokine-producing Subsets within Enriched CD154 Cells
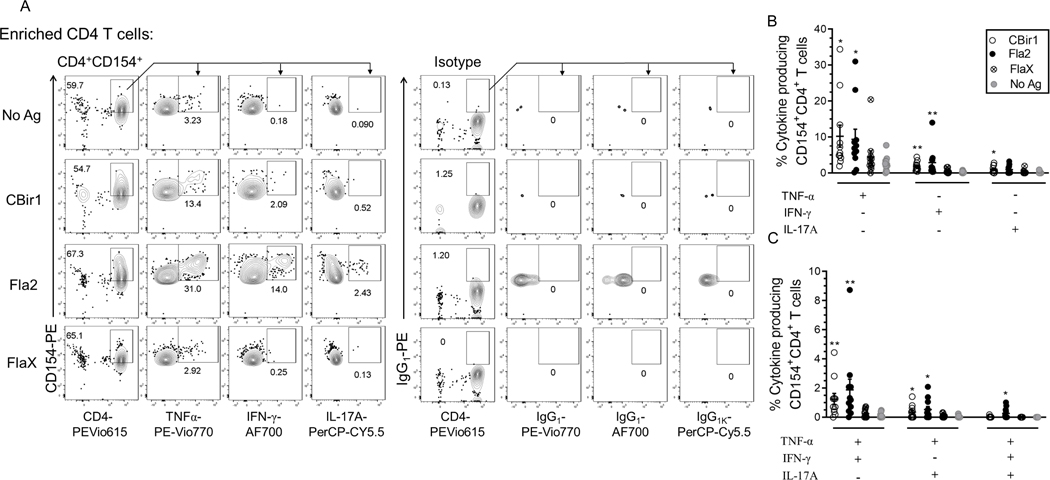
Antigen-reactive CD4+ T cells can produce cytokines upon exposure to their cognate antigen in the setting of antigen-presenting cells. Using a combination of CD154 enrichment and multiparametric analysis of cytokines by flow cytometry we examined CD154+ enriched cells for cytokine production. Following short term antigen stimulation, the surface-stained CD154-enriched cells were permeabilized, fixed and intracellularly stained for CD154, IFN-γ, TNF-alpha and IL-17A. Subsets of cytokine-producing CD154+CD4+ T cells were detected at varying numbers with the highest response to C. albicans (Supplemental Fig. 1B). The flagellin antigens CBir1, Fla2 and FlaX also induced cytokine production in smaller percentages of the CD154+CD4+ T cells (Fig. 2A). These data show that the CD4 T cells enriched for CD154 expression have both cytokine-producing and cytokine-nonproducing CD4 cells.
Figure 2. Flagellin antigen exposed CD4+ T cells analyzed for cytokine expression using the CD154+ enrichment assay.
(A) Representative contour plots of TNF-α, IL-17A and IFN-γ producing cells among CBir1 and Fla-2 exposed CD154+CD4+ T cells with (CD154 and cytokine) isotype control. (B) Frequency of CBir1-, Fla2- and FlaX-exposed IFN-γ+, IL-17A+ and TNF-α+ single positive CD154+CD4+ T cells among Crohn’s patients (n = 10). (C) Frequency of CBir1-, Fla2- and FlaX-exposed TNF-α+IL-17A+ and TNF-α+IFN-γ+ double positive CD154+CD4+ T cells among Crohn’s patients (n = 10). Bars represent the means ± SEM of ten independent experiments; nonparametric Kruskal- Wallis test with Dunn’s multiple comparison. *p<0.05, **p<0.01.
Among these small fractions of cytokine producing Ag-specific CD154+CD4+ T cells there were significantly higher percentages of Cytostim-stimulated and CBir1-, Fla2- and C. albicans-specific TNF-α, IFN-γ and IL-17A, single positive CD154+CD4+ T cells compared to unstimulated cells. (Fig.2B and Supplemental Fig. 1C). CD patients’ (n = 11) response to FlaX showed only a marginal increase in the fraction of TNF-α+, IL-17A+, IFN-γ+ single positive CD154+CD4+ T cells versus unstimulated cells (Fig. 2B).
Most of the patient samples also contained double-cytokine positive antigen-reactive cells. The percentage of TNF-α+ IFN-γ+ and TNF-α+ IL-17A+ double positive CD154+CD4+ T cells following exposure to CBir1, Fla2, Cytostim and C. albicans was significantly increased in the CD patients (n = 11) (Fig 2C and Supplemental Fig. 1D). A significantly high percentage of Fla2, C. albicans, and Cytostim-exposed triple cytokine positive (TNF-α, IL-17A, IFN-γ) CD154+CD4+ T cells were also detected in the CD patients. Triple cytokine positive CD154+CD4+ T cells were detected in only 1 of the patients stimulated by FlaX and 3 of the patients stimulated by CBir1. These data show that cytokine-producing flagellin-specific CD4+ T cells are heterogeneous minority fraction of the total CD154+CD4+ T cells population within Crohn’s peripheral blood.
Identifying Factors Contributing to CD154 Expression in the Absence of Antigen Stimulation
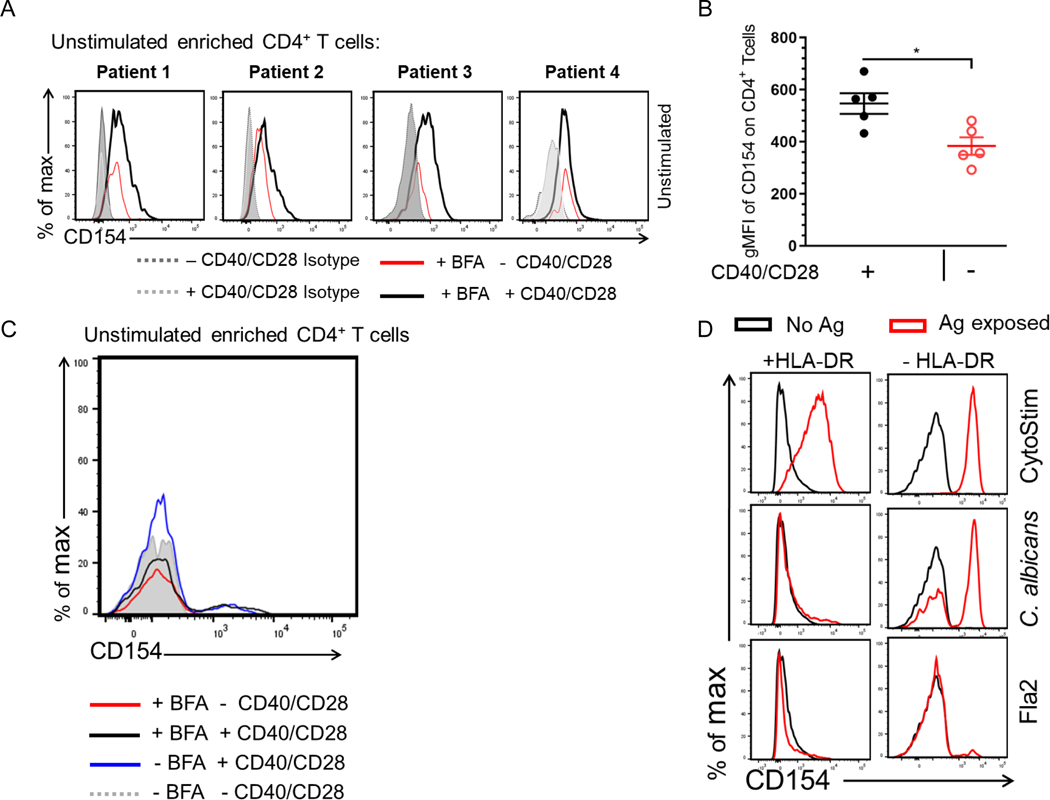
We found that a large proportion of the antigen exposed CD154+ enriched cells failed to produce cytokine during the incubation. It is unclear whether these cytokine-nonproducing cells are antigen-reactive clonotypes with less affinity for the antigen, are antigen reactive clonotypes at different phases of reactivity regardless of affinity, have pre-existing CD154 expression or have antigen-independent CD154 expression. To determine Ag-specific reactivity independent of cytokine production we analyzed the effects of culture conditions on CD154 expression among negative control cells. PBMCs from a cohort of Crohn patients (n = 4) were incubated for 7hr in the presence or absence of anti-CD40, anti-CD28 and Brefeldin A (added 3hr before harvest). Cells were enriched and stained for CD154 expression as discussed. All four donors showed that CD154 expression was significantly induced in comparison to isotype control. Moreover, CD4 cells that were cultured in the presence of anti-CD40, anti-CD28 also had a significantly higher expression of CD154 in comparison to cells that were cultured in the absence of anti-CD40 and anti-CD28 (Fig. 3A and Fig. 3B). On the other hand, Brefeldin A on its own did not appear to affect CD154 detection (Fig. 3C). The addition of anti-CD40 and anti-CD28 antibody to our assay has been shown to be necessary to prevent surface CD154 downregulation and to enhance short term T cell activation, respectively [16]. Our data indicate that the addition of these factors contribute to an increase background CD154 expression enough to capture these cells on the enrichment column.
Figure 3. Identifying factors that contribute to increase CD154 background on unstimulated cells.
(A) Unstimulated PBMCs were incubated for 7hr in the presence or absence of anti-CD40/anti-CD28 with BFA. Cells were enriched and stained for CD154 expression. Histogram plots of 4 donors showing CD154 expression among viable CD4+ T cells. (B) Scatter plot represents gMFI of CD154 expression in CD14−CD20−CD8−CD4+ T cells incubated with (black circle) or without anti-CD40/anti-CD28 (red circle) in the presence of BFA. Bars represent the means ± SEM of five independent experiments using different Crohn’s patients (n = 5); Mann-Whitney U comparison. *p<0.05. (C) Histogram of CD154 expression of a representative Crohn’s unstimulated CD4+ T cells incubated for 7hr with or without anti-CD40/anti-CD28 in the presence or absence of BFA. (D) Antigen exposed PBMCs from one active Crohn’s patient cultured in the presence of anti–HLA-DP-DR-DQ.
To examine whether CD154 expression in our system has antigen-dependent and independent contributions, we repeated our in vitro stimulation during blockade of antigen-presenting cell interaction with CD4 cells. C. albicans and flagellin antigens-exposed PBMCs treated with the pan-HLA-DR blocking antibody showed drastically decreased CD154 expression; the response to flagellin antigens: Fla2 (Fig. 3D), CBir1 and FlaX (not shown) was eliminated. A similar decrease was not seen for activation by CytoStim which does not depend on antigen presentation by an antigen presenting cell. These results suggest that while there is CD154 expression due to culture conditions, the majority of CD154 expression does seem to be related to antigen exposure and reveals a heterogeneity of CD4 response.
Characterizing the Subset of Cytokine-producing Flagellin-specific CD4 T cells
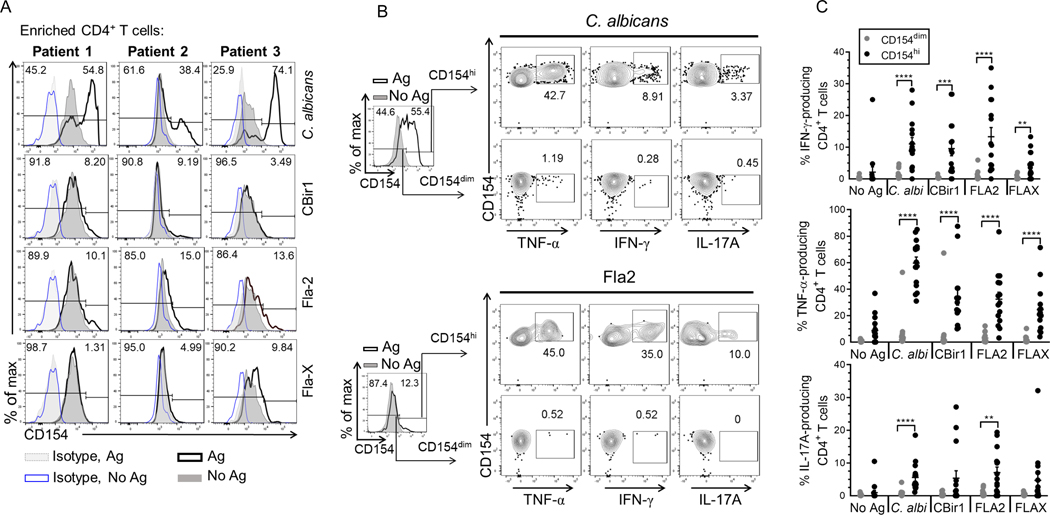
CD154-enriched cells following antigen exposure contain a large proportion of cells that overlap with CD154-enriched cells that were not incubated with antigens despite our exclusion criteria to remove doublets, dead cells, CD8+ T cells and non-lineage (CD14+, CD20+) cells prior to gating on CD4+ lymphocytes (Fig.4A). This was particularly important for studying flagellin-reactive T cells which were typically of lower number though equivalently high CD154 mean expression. We developed a gating strategy to eliminate these non-target cells for optimal detection of presumed antigen induced CD154+CD4+ events using the powerful stimulation by C. albicans antigen. Using a cohort of Crohn’s patients (n = 3), CD154 expression on C. albicans-exposed CD154+ enriched cells far exceeded that of the respective isotype stained control samples (without and with antigen exposure) confirming that little CD154 expression is due to nonspecific antibody staining (Fig. 4 and Supplemental Fig. 2A). Histogram plots of CD154 expression by C. albicans-exposed CD4+ T cells often show a bimodal distribution, we designated these two distinct CD154 expressing populations as CD154dim and CD154highCD4+ (Fig. 4A). Further examination of CD154 expression using histogram plot overlays for C. albicans-exposed and unexposed CD4+ T cells (and respective isotype controls) show that the C. albicans-exposed CD154dimCD4+ T cell population was completely overlapped by the antigen unexposed CD4+ T cells population. However, a relatively small amount of antigen-unexposed CD4+ T cells expressing CD154 (above the 97.5% gradient for CD154 staining) overlapped with the C. albicans-exposed CD154highCD4+ cell population). This suggested that following antigen exposure, CD4 cells could be divided into strata based on CD154 expression in the negative control cells (no added antigen). We have defined the CD154dim cells as those cells below the 97.5% proportion in antigen unexposed CD154+CD4+ T cells and the CD154hi cells which are cells above the 97.5% proportion of the antigen unexposed CD154+CD4+ T cells. Analysis of histogram plot overlays of CD154 expression by CytoStim-stimulated and unstimulated CD4+ T cells (and respective isotype controls) showed that there was also some overlap of CD154 expression between CytoStim-stimulated and unstimulated CD4+ T cells (Supplemental Fig. 2A).
Figure 4. Defining the cytokine-producing subset within the CD154+ CD4+ T cells following antigen exposure.
(A) Representative histogram overlay plots for CD154 expression among Crohn’s patients (n = 3). Histogram overlay plots are shown with isotype controls for antigen exposed cells (grey dotted line) and unexposed cells (blue solid line). CD154 expression for antigen unexposed cells (dark grey solid line) and antigen exposed cells (black solid line). (B) Representative flow plots of cytokine production by CD154 expressing cells. Histogram overlay plots are shown with unexposed cells (dark grey solid lines) and the antigen exposed cells (solid black line). Contour plots of TNF-α, IL-17A, IFN-γ production by CD154dim/hiCD4+ T cells following C. albicans, or Fla-2 exposure. (C) The cumulative frequency of TNF-α, IFN-γ, IL-17A produced by CD154dim (grey circle) or CD154hi (black circle) CD4+ T cells following antigen exposure among Crohn’s patients (n = 15). Bars represent the means ± SEM. of fifteen independent experiments; Mann Whitney test. **p<0.01, ***p<0.001, ****p<0.0001.
Therefore, we defined a positive antigen-specific response as that level of CD154 expression following Ag exposure above the 97.5% distribution of CD154 expression in the antigen unexposed cells. Using a gating scheme where we exclude the cells below the 97.5% proportion of unexposed CD154+ cells, we next examined flagellin-exposed cells among a group of CD patients (n = 3). Histogram plot overlays of unexposed and flagellin antigen-exposed cells show that above the 97.5% proportion of unstimulated CD154+ cells were a small distinct population of CD154high cells (Fig. 4A). Crohn’s patients had variable responses to CBir1, Fla2 and FlaX with reactivity to at least 2 of the 3 flagellin antigens. Overall, using a gating scheme that exclude cells below the 97.5% proportion of unstimulated CD154+ cells allowed for detection of a cell subset where flagellin antigen specific CD154+CD4+ T cells likely reside.
To further validate the exclusion of cells that fall below the 97.5% proportion of antigen unexposed CD154+ cells, we examined cytokine production by antigen-exposed cells that fall below the 97.5% (CD154dim) and above 97.5% (CD154hi) portion of unexposed CD154+CD4+ T cells (Fig. 4B; C. albicans and Fla2) among 15 active Crohn’s patients, we found that the antigen-exposed CD154hi cells produce significantly higher percentages of TNF-α, IFN-γ and IL-17A in comparison to CD154dim cells (Fig. 4C). Interestingly, CytoStim-stimulated CD154dimCD4+ T cells and CD154hiCD4+ T cells displayed similar levels of IFN-γ and IL-17A expression (Supplemental Fig. 2B and Fig. 2C).
Using another activation marker, CD69, along with CD154 induction did not improve the identification of strictly cytokine-producing cells following antigen exposure. We found that following stimulation CD154dimCD4+ T cells and CD154hiCD4+ T cells highly expressed CD69 (Supplemental Fig 3A). However, flagellin- and C. albicans-exposed CD69+CD154hiCD4+ T cells produce substantially higher TNF-α, IFN-γ and IL-17A (data not shown) cytokines in comparison to CD69+CD154dimCD4+ T cells but only in a subset of the CD69+CD154hi CD4+ T cells. In contrast, CytoStim-exposed CD69+CD154hiCD4+ T cells produce nearly similar levels of cytokines, particularly IFN-γ and IL-17A (data not shown) in comparison to CD69+CD154dimCD4+ T cells (Supplemental Fig 3B). Together these data demonstrate that including a criterion that removes the cells that fall below the 97.5% CD154 expression level in the negative control allowed us to focus on a subset of T cells that included the cytokine-producing CD4 cells. Including an additional activation marker did not help in further defining this set of cytokine-producing cells.
We also studied whether the CD154 enriched cells were uniformly effector memory cells and whether cytokine production was confined to a particular CD4 phenotype TEM (CD45RO+ and CD62L−), TCM (CD45RO+ and CD62L+), or Tnaive (CD45RO− and CD62L+) (Fig.5). We compared our antigen exposed CD154dim to CD154high populations and found that TEM cells dominated the CD4CD154high subset following exposure to antigen and that Tnaïve and TCM cells were the most abundant in the CD154dim population (Fig 5A). Cytokine production by the antigen exposed CD154hiCD4+ T cells was almost exclusively limited to the TEM (Fig. 5B). In contrast, following polyclonal stimulation, we found no difference in the percentage of central memory, effector memory and naïve cells phenotype among the CD154hiCD4+ and CD154dimCD4+ T cells (Supplemental Fig. 2D). In addition, we found no difference in cytokine production by cytostim-stimulated CD154hiCD4+ T cells among the different CD4 subsets (Fig. 5B).
Figure 5. The expression of CD62L and CD45RO by antigen exposed CD154dim/hiCD4+ T cells was analysed by flow cytometry.
(A) The percentage of CD62L and CD45RO expression by antigen exposed CD154dim/hiCD4+ T cells. Bars represent the means ± SEM of eight independent experiments; two-way ANOVA Dunnett’s multiple comparison tests. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. (B) Representative contour plots of cytokine production by CD154hi cells among different CD4 subsets following C. albicans exposure. (C) Cumulative frequency of TNF-α, IFN-γ, IL-17A produced by CD154hiCD4+ T cells subsets following antigen exposure among active Crohn’s patients (n = 8). Bars represent the means ± SEM of eight independent experiments; nonparametric Kruskal-Wallis test with Dunn’s multiple comparison. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Flagellin-specific T cells in Crohn’s Disease Patients and Healthy Controls.
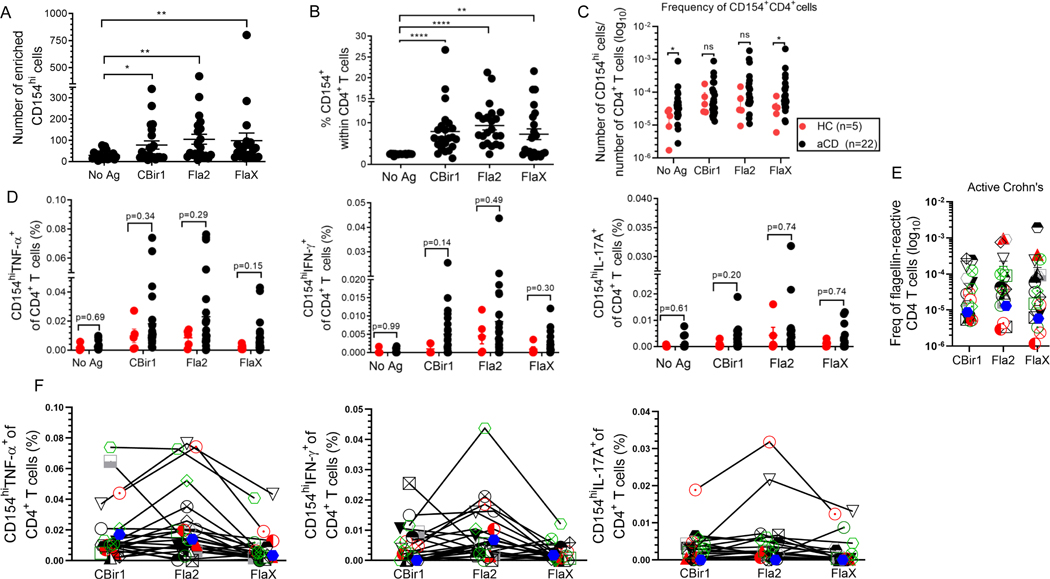
Among patients with active Crohn’s disease (n = 22) a significantly high numbers and percentages of CD154hiCD4+ T cells was observed following flagellin antigen exposure (Fig. 6A and Fig. 6B). Similar data was found for C. albicans- exposed and CytoStim-stimulated CD154hiCD4+ T cells (Supplemental Fig. 4A). The absolute frequencies of CD154hiCD4+ T cells in the peripheral blood of active Crohn’s (based on the total number of CD4+ T cells applied on the column and excluding the non-target cells) ranged between 1 and 100 (CBir1), 200 (Fla2), and 200 (FlaX) CD154hi cells per 1 × 105 CD4+ T cells (Fig. 6C). As expected, there were much higher frequencies for C. albicans-reactive T cells (8 – 900) and CytoStim stimulated cells (32 – 3200) CD154hi cells per 105 CD4+ T cells (Supplemental Fig. 4C). Reactivity to the tested flagellin antigen were detected in a majority of the active CD patients. To determine whether flagellin T cells response was CD specific we evaluated the frequencies of flagellin reactive CD154hiCD4+ T cells among non-IBD healthy control patients (n = 5) and active Crohn’s patients (n = 22). The frequencies of flagellin reactive CD154hiCD4+ T cells were higher in active CD in comparison to healthy control subjects (Fig. 6C). In addition, these flagellin-specific CD154hiCD4+ T cells displayed a trend towards elevated percentage of TNF-α, IFN-γ and IL-17A compared to those from the healthy donors (Fig. 6D).
Figure 6. Flagellin-specific CD4+ T cells can be detected in Crohn’s and healthy patients. (A) CD4+ T cell responses to antigen exposure were analyzed in active Crohn’s (n = 22).
Number of enriched CD154hi cells active CD patients (n = 22). (B) Percentage of CD154hi cells among CD4+ T cells from active Crohn’s (n = 22) with expression above the background level of approximately 2.50%. (C) Frequencies of CD154hiCD4+ T cells in peripheral blood of active Crohn’s (black dots; n = 22) and healthy (red dots; n = 5) patients were calculated from the number of CD154hi cells obtained after enrichment normalized to the total number of CD4+ T cells applied on the column. (D) Frequency of cytokine producing cells among active Crohn’s (n = 22) and healthy (n = 5) patients were calculated from the total number of cytokine positive cells normalized to the total number of CD154hi cells obtained after enrichment. Bars represent the means ± SEM of five-twenty-two independent experiments; Mann Whitney test. *p<0.05, **p<0.01, ****p<0.0001. (E).The frequency of flagellin-specific CD4 T cells (stimulated sample minus Ag unexposed control for CD154hi cells; background-subtracted). (F). Comparison of CD154hiCD4+ T cells cytokine reactivity to different flagellin antigens within the same active Crohn’s patient (n = 22). Each symbol represents one patient (lines link result within the same active Crohn’s patient).
Although Hegazy et al., recently reported that there are reduced frequencies of circulating C. albicans-reactive CD154+CD4+ T cells and increased frequencies of SEB-stimulated CD154+CD4+ T cells in Crohn’s patients compared to healthy controls, we found no significant difference in the frequencies of C. albicans-reactive CD154hiCD4+ T cells between Crohn’s and healthy control patients. We however did observe a higher frequency of CytoStim-stimulated CD154hiCD4+ T cells among active Crohn’s patients compared to those from healthy donors (Supplemental Fig. 4C and Supplemental Fig. 4D).
We also noted that Crohn’s patients did not have uniform patterns of response to all antigens. To determine whether a patient with high T cell reactivity to one flagellin antigen had a similar high reactivity to another flagellin antigen we examined the responses to CBir1, FlaX and Fla2 among active Crohn’s subjects (Fig. 6E and Fig. 6F). Even though several patients had a strong response to all the tested flagellin antigens there were some patients that had a strong response to only one or two of the tested flagellin antigens. High levels of TNF-α-, IFN-γ- and IL-17A were not seen in response to all tested flagellin antigens among patients reactive to all these antigens (Fig. 6F). Likewise, patients reactive to one or two flagellin antigen did not produce equally high level of TNF-α-, IFN-γ- and IL-17A in response to those antigens (Supplemental Fig. 4E). These results demonstrate that flagellin-specific CD4+ T can be detected in Crohn’s as well as some healthy patients.
Discussion
Gut microbial Ag-specific CD4 T cells play a key role in intestinal inflammation in experimental models [1, 2]. The association of antibodies against flagellin antigens in Crohn’s disease is well known, but less is known about aberrant T cell responses to the same immunodominant antigens. A few translational studies have attempted to functionally characterize microbial reactive CD4+ T cells in IBD patients [10, 24]. Recently, Hegazy, A.N., et al. used the CD154 expression in CD4 cells to demonstrate that microbial-reactive CD4+ T cells can be detected in the peripheral blood and intestinal tissues of healthy adults as well as IBD patients. These studies used whole bacterial lysates and not purified recombinant bacterial antigens, including flagellins, so specific antigen reactivity could not be reported [23]. Other studies characterizing flagellin-reactive CD4+ T cells have utilized other methods that rely on antigen-induced proliferation (CFSE and [3H] thymidine incorporation) or induction of activation markers like OX40 and CD25 [10, 24]. The differences in these assays include an extended antigen exposure time (16hr to 7 days) necessary for stimulation which can miss early events like cytokine production and possibly overlook the earliest-responding CD4 clonotypes. So, some of the gaps in the knowledge of microbial reactive CD4 cells in Crohn’s disease (and healthy controls) include identifying candidate clonotypes reactive to purified immunodominant microbial antigens relevant to Crohn’s disease and characterizing the phenotype of these CD4 cells and the heterogeneity of the response itself among Crohn’s patients.
In this report we have measured the presence and functionality of flagellin-reactive CD4+ T cells in Crohn’s disease patients with the help of an antigen-reactive T cell enrichment approach. PBMCs from donors were stimulated for only 7hrs and cells were enriched for CD154 expression. CD154 is upregulated on T cells when T cells recognize peptide antigen/MHC complexes through αβ-TCR and receive co-stimulation through CD28 binding to CD80 and CD86. CD154 is transiently expressed on T cells but its interaction with CD4 is necessary for generating Th1 CD4+ memory T cells. CD154 enrichment facilitates the ex vivo analysis of the Ag-specific T cells using CD154 expression only or in combination with cytokine secretion [21]. By using this method, we show that short term induction of (intracellular) CD154 is sensitive enough to detect small numbers of circulating flagellin antigen reactive memory CD4+ T cells from Crohn’s disease patients.
However, our study revealed an increase in CD154 expression in CD4 cells not exposed to added antigens using this method. Only one other group has reported CD154 background expression in CD4 cells that were not exposed to exogenous antigen during incubation; this group used CD154 staining during culture conditions along with anti-CD40 and anti-CD49d antibodies rather than our CD154 enrichment strategy [19]. Using an intracellular CD154 staining approach Frentsch, M., et al. found no increase in CD154 expression in control cultures (no added antigen) following 6h culture with anti-CD40 mAb alone [22]. However, as shown by our data we found that adding anti-CD40 and anti-CD28 mAb leads to an “antigen-independent” stimulation of CD154 expression within our control cultures. The addition of anti-CD40 antibody to our assay is necessary the block the binding of CD154 (CD40L) with CD40 which prevents CD154 internalization and degradation. CD28 has a role in initiating T cell activation by binding CD80/86 on APC. The added anti-CD28 antibody in our assay will bind CD28 and stimulate T cells without CD80/86 on APC leading to enhanced short-term T cell activation. Perhaps the addition of anti-CD40 and anti-CD28 antibody to our culture enhances the activation of T cells that interact with APCs that are preloaded with other antigens and are stimulated but are unable make sufficient cytokine. These CD154 expressing cells need to be accounted for when trying to identify actual antigen-specific clonotypes.
One way to account for antigen-reactive CD4 cells among the CD154 expression increased by the culture conditions themselves was to measure another cell response that was associated with antigen exposure alone, in this case intracellular cytokine production was a useful discriminating factor. Using an additional activation marker like CD69 along with CD154 expression did not improve the ability to identify the specific subset of cytokine-producing antigen reactive cells. We found that the cytokine producing CD4 cells could always be found in that fraction of CD154 positive cells above the top 2.5% of CD154 MFI in the control cultures. No cytokine co-expression was ever found below this gate. In fact, we took this as the gating strategy to identify the antigen-reactive cells in the antigen-added cultures; the percentage of CD154 expressing cells from these antigen-exposed cultures ranged from 3.26% to 26.7 % CBir1-reactive, 4.38% to 19.8 % Fla2-reactive, 3.1% to 21.6 % FlaX-reactive, 43% to 77.1% C. albicans-reactive and 35.8% to 83.4% CytoStim-stimulated cells.
After accounting for non-specific CD154 expression and using cytokine production to characterize antigen reactivity within this enriched population, we can identify distinct populations of flagellin-Ag-specific CD4+ T cell that range between 1 and 200 CD154hi cells per 105 CD4+ T cells among the total enriched CD154 cells. The cytokine profile of flagellin antigen reactive CD154hiCD4+ T cells in Crohn’s patients is consistent with previous studies reporting that FlaX and A4-Fla2 specific CD4+ T cells in Crohn’s patients can display a Th1 (IFN-γ), Th17 (IL-17A) and Th1/Th17 (IFN-γ+ IL17-A+) cytokine profiles [10, 18, 24]. We demonstrated that a higher frequency of flagellin antigen reactive CD4+ T CD154hi expressing cells were found among active Crohn’s patients compared to non-IBD control donors. We also see a higher percentage of cytokine producing cells among some of the active Crohn’s compared to non-IBD control patients. Unlike previous studies where background (CD154 cells among control) was subtracted to show Ag-specific (CD154+) T cell response only among antigen stimulated cells we have shown the frequencies of CD154 without background subtraction. Interestingly, PBMC from Crohn’s patients that were not exposed to any of our tested antigens exhibited a higher frequency of CD154hiCD4+ T cells compared to antigen unexposed cells from healthy controls. This could imply that there are more CD4 T cells primed by other antigens or stimuli, possibly related to increased gut permeability, other than those we have tested in the active Crohn’s compared to healthy patients. Further examination of cytokine production by these non-specifically activated CD154hi CD4T cells from CD and healthy control patients showed that there is no significant difference in cytokine production associated with increased CD154 expression.
Among the Ag-stimulated CD154 expressing T cells that produced cytokine we observed a higher percentage of cytokine producing cells among the active Crohn’s compared to non-IBD control patients. The frequency of cytokine producing flagellin-specific CD4 T cells ranged from 0.00–0.08% (TNF-α), 0.000–0.05% (IFN-g) and 0.00–0.035% (IL-17A). These frequencies are well within the expected range of microbial Ag-specific T memory cells which has been shown to be typically below 1% in the absence of acute infections [16 and 23]. Hegazy et al using the CD154 approach, previously demonstrated that the frequency of microbiota-reactive memory cells CD154+CD4+ TNF-α+ cells within PBMC of healthy donors and IBD patients can ranged from 0.0–0.025% (S. Typhimurium) and 0.0–0.08% (C. difficule) [23].
Not all Ag-stimulated CD154 expressing T cells from our donors produced cytokines (TNF-α, IFN-γ and IL-17A) and in fact, there was a higher percentage of CD154 cells that did not produce these cytokines. It is possible that these CD154+ cells in our gating strategy may produce other effector cytokines, since we were able to measure IL-13, IL-5, IL-4, IL-22 and IL-10 by multiplex cytokine assay of culture supernatant (data not shown) and other cytokines may need more time to be produced [16,18]. It is also possible that these are flagellin-specific cells but with lower affinity TCR clonotypes to different peptide-MHC complexes or are at a different status in terms of their overall ability to respond to antigen.
Futhermore, the magnitude and consistency of response to the different flagellin antigens (CBir1, Fla2, FlaX) is not predictable among patients. While some patients responded to all three flagellins, there are patients that responded to only one flagellin antigen. Among patients with high reactivity to all antigens we see that all the flagellins do not produce high levels of cytokine. These findings suggest that there may not be a strict hierarchy of the strength of response for a particular flagellin antigen within and among patients.
In summary, our data show that among the distinct CD154hi flagellin- specific T cells are a subset of cells that produce the relevant TNF-a, Th1 and Th17 cytokines that are involved in CD inflammation [25]. Among the key points of this study is that in the search for antigen specific CD4 clonotypes, especially when single cell isolation is required for study, then attention to defining antigen-reactive (as opposed to non-specifically activated) T cells can require additional features. Another point is that the cytokine producing cells that we are identifying as antigen-specific may be only a subset of actual flagellin-reactive CD4 cells. The others that we can see by flow cytometry may be producing other cytokines, needing more time to produce their cytokine, may have TCRs with varying affinities for the antigen peptide-MHC complex, or may be at different cell phases that affect their ability to respond.
Future studies evaluating the clonality, phenotypes, transcriptomes and gene signatures of these cytokine-producing CD4 cells and the cytokine-negative CD154hi flagellin-specific T cells from PBMC and LPMC populations will be valuable towards the understanding of functional differences between flagellin-reactive cells in Crohn’s and healthy controls. Our previous work had found TCRβ CDR3 sequences in both CD4 cells from PBMC and LPMC populations in the same patient, supporting shared clonotypes in the blood and lamina propria in Crohn’s disease. Our current report was an approach to more thoroughly interrogate the CD154 expression method for identifying antigen-reactive cells within PBMC as a way to identify these cells. Unfortunately, the CD154 assay requires many more CD4 T cells [16, 18, 19 and 22] than we can generally obtain from multiple endoscopic biopsies (we prefer to use biopsies to surgically resected bowel specimens as the latter typically come from patients who have been concurrently exposed to excessive immunosuppression). We are working on improving methods to compare flagellin reactivity between blood and gut mucosa CD4 cells within and among Crohn’s and control subjects to further extend our knowledge of the interplay of flagellin-reactive cells and their role in disease severity, activity, and potential for therapeutic targeting.
Supplementary Material
Key Messages.
Only a small subset of flagellin-induced CD40LhiCD4 cells express cytokines.
This CD40LhiCD4 cytokine production is restricted to T effector memory cells.
Acknowledgements
We would like to thank Dr. Charles O. Elson and Mr. L. Wayne Duck (University of Alabama at Birmingham) for providing us with the flagellin antigens, Dr. Frances E. Lund (University of Alabama at Birmingham) for granting us access to the MagPix instrumentation, and Dr. Davide Botta (University of Alabama at Birmingham) for running the Luminex multiplex assays. We thank the Department of Medicine and the Program in Immunology at the University of Alabama at Birmingham.
This study was supported by a grant from the Department of Veterans Affairs CX0001530 and Impact funds from UAB.
Abbreviations used in this article:
- IBD
Inflammatory bowel
- (a)CD
(active) Crohn’s disease
- HC
healthy control
- AIM
Activation Induced Marker
- TEM
effector memory T cells
- TCM
central memory T cells
- BFA
Brefeldin A
- Cyto
CytoStim
- C. albi
Candida albicans
- SEB
Staphylococcus aureus enterotoxins
Footnotes
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Sartor RB Microbial influences in inflammatory bowel diseases. 2008. Gastroenterol.134 (2): 577–594. [DOI] [PubMed] [Google Scholar]
- 2.Feng T, Wang L, Schoeb TR, Elson CO and Cong Y. 2010. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J. Exp. Med 207 (6): 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Madeline F. and Hershberg RM 2004. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Invest 113(9): 1296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen C, Landers CJ, Derkowski C, Elson CO and Targan SR. 2008. Enhanced CBir1-specific innate and adaptive immune responses in Crohn’s disease. Inflamm. Bowel Dis 14 (12): 1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, Landers CJ, T Abreu-Martin M, Rotter JI, Yang H. and Targan SR. 2004. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterol. 126 (2): 414–424. [DOI] [PubMed] [Google Scholar]
- 6.van Schaik FD, Oldenburg B, Hart AR, Siersema PD, Lindgren S, Grip O, Teucher B, Kaaks R, Bergmann MM, Boeing H, Carbonnel F, Jantchou P, Boutron-Ruault M-C, Tjønneland A, Olsen A, Crowe FL, Peeters PHM, van Oijen MGH and Bueno-de-Mesquita HB 2013. Serological markers predict inflammatory bowel disease years before the diagnosis. Gut. 62 (5): 683–688. [DOI] [PubMed] [Google Scholar]
- 7.Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO and Hershberg RM 2005. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterol. 128 (7): 2020–2028. [DOI] [PubMed] [Google Scholar]
- 8.Elkadri AA, Stempak JM, Walters TD, Lal S, Griffiths AM, Steinhart AH and Silverberg MS 2013. Serum antibodies associated with complex inflammatory bowel disease. Inflamm. Bowel Dis 19 (7): 1499–1505. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Pendegraft AH, Byrne-Steele M, Yang Q, Wang C, Pan W, Lucious T,T Seay, Cui X, Elson CO, Han J, and Mannon PJ 2018. Expanded TCRbeta CDR3 clonotypes distinguish Crohn’s disease and ulcerative colitis patients. Mucosal Immunol. 11 (5): 1487–1495. [DOI] [PubMed] [Google Scholar]
- 10.Calderón-Gómez E, Bassolas-Molina H, Mora-Buch R, Dotti I, Planell N, Esteller M, Gallego M, Martί M, Martίnez-Torró C, Ordás I, Singh S, Panés J, Benίtez-Ribas D, and Salas A. 2016. Commensal-Specific CD4(+) Cells From Patients With Crohn’s Disease Have a T-Helper 17 Inflammatory Profile. Gastroenterol. 151 (3): 489–500 e3. [DOI] [PubMed] [Google Scholar]
- 11.Elias G, Ogunjimi B. and Van Tendeloo V. 2020. Activation-induced surface proteins in the identification of antigen-responsive CD4 T cells. Immunology Letters. 219:1–7. [DOI] [PubMed] [Google Scholar]
- 12.Zaunders JJ, Munier ML, Seddiki N, Pett S, Ip S, Bailey M, Xu Y, Brown K, Wayne BD, Kim M, Rose R.d., Kent SJ, Jiang L, Breit SN, Emery S, Cunningham AL,Cooper DA and Kelleher AD 2009. High levels of human antigen-specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). J. Immunol 183 (4): 2827–2836. [DOI] [PubMed] [Google Scholar]
- 13.Bacher P, Kniemeyer O, Teutschbein J, Thön M, Vödisch M, Wartenberg D, Scharf DH, Koester-Eiserfunke N, Schütte M. Dübel S, Assenmacher M, Brakhage AA and Scheffold A. 2014. Identification of immunogenic antigens from Aspergillus fumigatus by direct multiparameter characterization of specific conventional and regulatory CD4+ T cells. J. Immunol 193 (7): 3332–3343. [DOI] [PubMed] [Google Scholar]
- 14.Battaglia E, Biancone L, Resegotti A, Emanuelli G, Fronda GR, and Camussi G. 1999. Expression of CD40 and Its Ligand, CD40L, in Intestinal Lesions of Crohn’s Disease. Am. J. Gastroenterol 94 (11): 3279–3284 [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Colpaert S, D’Haens GR, Kasran A, Boer M. d., Rutgeerts P, Geoboes K. and Cueppens JL 1999. Hyperexpression of CD40 Ligand (CD154) in Inflammatory Bowel Disease and Its Contribution to Pathogenic Cytokine Production. The J. Immunol 163 (7): 4049–4057. [PubMed] [Google Scholar]
- 16.Bacher P, Schink C, Teutschbein J, Kniemeyer O, Assenmacher M, Brakhage AA and Scheffold A. 2013. Antigen-reactive T cell enrichment for direct, high-resolution analysis of the human naive and memory Th cell repertoire. J. Immunol 190 (8): 3967–3976. [DOI] [PubMed] [Google Scholar]
- 17.Dan JM, Lindestam Arlehamn CS, Weiskopf D, da Silva Antunes R, Havenar-Daughton C, Reiss SM, Brigger M, Bothwell M, Sette A. and Crotty S. 2016. A Cytokine-Independent Approach To Identify Antigen-Specific Human Germinal Center T Follicular Helper Cells and Rare Antigen-Specific CD4+ T Cells in Blood. J. Immunol 197 (3): 983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chattopadhyay PK, Yu J, Roederer M. 2006. Live-cell assay to detect antigen-specific CD4+ T-cell responses by CD154 expression. Nat Protoc. 1(1):1–6. [DOI] [PubMed] [Google Scholar]
- 19.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005; 11(10): 1113–117. [DOI] [PubMed] [Google Scholar]
- 20.Walsh AJ, Bryant RV, and Travis SPL 2016. Current best practice for disease activity assessment in IBD. Nat. Rev. Gastroenterol. & Hepatol 13 (10): 567–579. [DOI] [PubMed] [Google Scholar]
- 21.Mueller M, Spangenberg HC, Kersting N, Altay T, Blum HE, Klenerman P, Thimme R. and Semmo N. 2010. Virus-specific CD4+ T cell responses in chronic HCV infection in blood and liver identified by antigen-specific upregulation of CD154. J. Hepatol 52 (6):800–811. [DOI] [PubMed] [Google Scholar]
- 22.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A. and Thiel A. 2005. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat. Med 11 (10): 1118–1124. [DOI] [PubMed] [Google Scholar]
- 23.Hegazy AN, West NR, Stubbington MJT, Wendt E, Suijker KIM, Datsi A, This S, Danne C, Campion S, Duncan SH, Owens BMJ, Uhlig HH, McMichael A, Bergthaler A, Teichmann SA, Keshav S. and Powrie F. 2017. Circulating and Tissue-Resident CD4(+) T Cells With Reactivity to Intestinal Microbiota Are Abundant in Healthy Individuals and Function Is Altered During Inflammation. Gastroenterol. 153 (5):1320–1337.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook L, Lisko DJ, Wong MQ, Garcia RV, Himmel ME, Seidman EG, Bressler B, Levings MK and Steiner TS 2020. Analysis of Flagellin-Specific Adaptive Immunity Reveals Links to Dysbiosis in Patients With Inflammatory Bowel Disease. Cell Mol. Gastroenterol. Hepatol 9 (3): 485–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omer OS, Powell N, and Lord GM 2020. Characterizing Innate Lymphoid Cell Phenotype and Function in Human Inflammatory Bowel Disease. In Methods in Molecular Biology. Clifton, N.J. 2121: 199–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.