Table 2.
List of phytochemicals showing therapeutic promise against PCa in preclinical model.
| Compound & chemical nature | Plant name and family | Mechanism of actions | In vitro | In vivo | Combination drug | References |
|---|---|---|---|---|---|---|
 Baicalein (Flavone) |
Scutellariabaicalensis (Lamiaceae) | Inhibited cell cycle regulatory proteins, G0/G1 arrest, CDK6, FOXM1, cyclin B, and Aurora B. Blocked cell proliferation through inducing apoptosis; reduced tumor overload in vivo. Blocked proliferation by inducing cell cycle arrest and apoptosis involving downregulating Ezrin expression. Downregulated cyclin D1, CDK4 while upregulating p53 and p21 levels. Decreased tumor overload in vivo by counteracting Ezrin function. It ameliorated benign prostate hyperplasia (BPH) through androgen-dependent apoptosis. Blocked prostate enlargement, production of DHT, and 5α-reductase activity type II in vivo. Increased Bax/Bcl-2 ratio, caspase-dependent apoptosis (Caspase 3, -8) and p-AMPK level, and reduced AR, PSA, PCNA, and Bcl-2 in vitro |
LNCaP, PC3 PC3 NA |
LNCaP xenograft PC3 xenograft Chemical (testosterone, DHT) induced BPH |
NA NA NA |
Yu et al. (2020) (Ma et al., 2020) (Jin and An, 2020) |
 Berberine (Isoquinoline alkaloid) Berberine (Isoquinoline alkaloid)
|
Berberis genus (Berberidaceae) |
Inhibits cell growth and induces apoptosis. It decreases transcriptional activity and signaling of AR induces AR protein degradation both in cells and tumor tissue. Metabolomics and network analyses suggested that berberine reversed the abnormality in metabolic biomarker expression by adjusting the perturbed metabolic pathways. Berberine inhibited p38 MAPK signaling to inhibit its downstream pro-oncogenic targets; inhibited tumor burden in vivo through inducing apoptosis. Inhibited benign prostatic hyperplasia induced by testosterone through inhibiting 5 alpha-reductase, AR, steroid coactivator-1 (SRC-1), and ERK signaling. Inhibited proliferation and growth in vitro and in vivo by inducing apoptosis, radio-sensitivity through inhibiting expression of VEGF and HIF1a. Acted in synergy with ionizing radiation. Inhibited tumor growth by facilitating p53 dependent and independent death and growth arrest at G0/G1 phase; enhanced p21 expression while decreasing cyclin D1, E and CDK 4, and -2 expression. |
LNCaP, 22Rv1, PC3, LAPC-4 C4–2B 22Rv1 RWPE-1 LNCaP, DU145 PC3, LNCaP, PWR1-E |
LNCaP grafted tumors 22Rv1 grafted nude mice Male Sprague–Dawley (SD) rats LNCaP grafted Balb/c nude mice PC3, LNCaP grafted Balb/c nude mice |
NA NA NA Ionizing radiation NA |
Li et al. (2011) (Li et al., 2017) (Youn et al., 2018) (Zhang et al., 2014) (Choi et al., 2009) |
 Betulinic Acid (BA) (Pentacyclic triterpene) |
Betula papyrifera (Betulaceae) |
Inhibited deubiquitinases to increase the levels of poly-ubiquitinated proteins and apoptosis in ex vivo and in vivo tumor model; lowered the levels of oncoproteins, cyclin D1 and AR protein. |
LNCaP, DU145, and PC3 |
TRAMP mice |
NA |
Reiner et al. (2013) |
 Capsaicin (Vanilloid type of compound) Capsaicin (Vanilloid type of compound)
|
Capsicum genus (Solanaceae) |
Oral administration increased the efficacy of radiotherapy and reduced the tumor burden; inhibited colony formation, NF-κB level, and DNA damage. Inhibited cell proliferation synergistically with docetaxel ex vivo and in vivo targeting PI3k/Akt/mTOR pathway. Overexpression of PTEN and inhibition of AMPK abrogated the functional synergy with docetaxel. Administration by oral route resulted in reduced metastatic potential, which was correlated with the reduction in p27/Kip1 expression and neuroendocrine differentiation in the tumor tissues; no toxicity to the liver or gastrointestinal tract was observed. Inhibited PC3 cell growth and proliferation both in vitro and in vivo. Antiproliferative effect on PCa cells was associated with increased p53, p21, and Bax level, and NFκB inhibition through suppression of IkBα degradation by blocking proteasome activity |
LNCaP, DU145, and PC3 LNCaP, PC3 PC3 PC3, DU145, LNCaP |
LNCaP grafted nu/nu athymic nude mice PC3 and LNCaP grafted athymic nude-Foxn1 (nu/nu) mice Transgenic adenocarcinoma of the mouse prostate (TRAMP) model. PC3 xenograft |
Increase radiosensitivity Synergizes docetaxel's activity in vitro and in vivo NA NA |
Venier et al. (2015a) (Sánchez et al., 2019) (Venier et al., 2015b) (Mori et al., 2006) |
 Delphinidin (Anthocyanin type of compound, specifically Anthocyanidin) Delphinidin (Anthocyanin type of compound, specifically Anthocyanidin)
|
Viola sp. (Violaceae)and Delphinium sp. (Ranunculaceae) |
Induced caspase-dependent apoptosis, G2/M arrest with reduction of p-IκB kinase γ; (NEMO), phosphorylation of NF-κB inhibitor y protein IκBα and phosphorylation of NF-κB/p65 at Ser536 and NF-κB/p50 at Ser529, and thus NF-κB/p65 nuclear translocation, and NF- κB DNA binding activity. Ameliorated tumor overload in vivo with a decrease in Bcl-2, Ki67, and PCNA levels. |
PC3, LNCaP, C4-2, 22Rv1 |
PC3 grafted nude mice |
NA |
Hafeez et al. (2008) |
 Fisetin (Flavonol) Fisetin (Flavonol)
|
Acacia greggii (Fabaceae) |
Reduced cell viability in vitro. Enhanced the ability of cabazitaxel to block cell proliferation through induction of apoptosis; reduced tumor growth, invasion, metastasis with reduction of PCNA, Ki67 levels while enhancing the level of Bax. Disrupted microtubule dynamics in PCa cells. Blocked PCa progression by inhibiting hyaluronan (HA) synthesis and degradation enzymes; increased the level of antiangiogenic high molecular mass hyaluronan (HMM-HA). Blocked tumor progression in TRAMP mice model and tumor xenograft studies. Blocked the expression and EGF-induced phosphorylation of Y-box binding protein-1 (YB-1) and markers of EMT (Vimentin, slug) while increasing E-cadherin to restrain EMT in vitro and in vivo. Prevented angiogenesis in vitro and in vivo by reducing VEGF endothelial NO synthase expression. Caused cell death by caspase-dependent apoptosis and increased Bax/Bcl-2 ratio and p53 level. Inhibited tube formation in HUVEC cells. Induced cell cycle arrest at G1 (strong) and G2/M (moderate) phases together with reducing cyclin D level. |
PrEC, 22Rv1, and C4-2, PC3M-luc-6, NCI/ADR-RES RWPE, NB11, NB26 RWPE-1, LNCaP, DU145, 22Rν1, PC-3, NB26 DU145, HUVEC |
22Rv1 and PC3 M-luc-6 grafted mice NB11 and NB26 grafted nude mice and TRAMP mice NB26 grafted nude mice Matrigel implanted Swiss albino mice |
Enhanced the efficacy of cabazitaxel NA NA NA |
Mukhtar et al. (2016) (Lall et al., 2016) (Khan et al., 2014) (Bhat et al., 2012) |
 Formononetin (Isoflavone derivative (O-methylated isoflavone)) Formononetin (Isoflavone derivative (O-methylated isoflavone))
|
Trifolium pretense (Fabaceae) | Inhibited cell proliferation by facilitating G1 arrest in PCa cells via downregulation of Akt/Cyclin D1/CDK4. Inhibited tumor overload in vivo. | PC3, DU145 | PC3 grafted nude mice | NA | Li et al. (2014) |
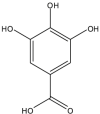 Gallic Acid (GA) (Polyphenolic compound) |
Vitis vinifera (Vitaceae) | Blocked PCa progression through inhibiting histone deacetylase 1, 2, and PCNA and upregulation of acetyl-p53. Enhanced intrinsic caspase-dependent apoptosis, DNA fragmentation with a decrease in mitochondrial membrane potential, and cyclin D1, -E1, and PCNA levels while upregulating p21/Waf1 level. Induced PCa cell death by apoptosis and reduced microvessel density and tumor overload in vivo. Inhibited tumor growth by apoptosis; reduced PCNA level, cell cycle markers cyclin B1, -E1, CDK2, -4, -6, and Cdc2 level. GA present in pomegranate peel extract reduced tumor burden in vivo accompanied by inhibition of TNF-α and VEGF expression. |
LNCaP, PC3 DU145, 22Rv1 NA PC3 |
PC3 grafted in BALB/c nu/nu male mice DU145, 22Rv1 grafted nude mice TRAMP mice PC3 xenograft |
NA NA NA NA |
Jang et al. (2020) (Kaur et al., 2009) (Raina et al., 2008) (Ma et al., 2015) |
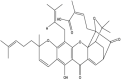 Gambogic Acid (Xanthone derivative) Gambogic Acid (Xanthone derivative)
|
Carciniahanburyi (Clusiaceae). |
Inhibited human umbilical vascular endothelial cell (HUVEC) proliferation, migration, invasion, tube formation, and microvessel growth at the nanomolar concentration in vitro. Reduced angiogenesis, VEGF-2, c-Src, focal adhesion kinase, and Akt level in vivo. |
PC3, HUVEC |
PC3 grafted SCID mice |
NA |
Yi et al. (2008) |
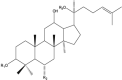 Ginsenosides (GN) (Triterpene saponin) |
Panax ginseng (Araliaceae). |
GN Rh2 blocked the growth of tumors developed with three different PCa cells by suppressing angiogenesis by inhibiting the expression of CNNM1, CD31, VEGF, and PDGF genes. GN-Rg3 showed synergistic activity with chemotherapeutics in killing PCa cells. Induced arrest in G0/G1 phase and apoptosis. Inhibited NF-κB, Bcl-2, IAP-1, XIAP, cyclin B, D1, E, and CDK 2, -4. Combination with docetaxel and cisplatin exhibited functional synergy in blocking PCa. GN-Rh2 blocked tumor growth and invasiveness. Activated TGF-β receptor signaling, regulators of cell cycle mediators, and MMPs. Downregulated cyclin D1, B1, MMP2/9 while increasing the level of pSMAD2 and p27. |
LNCaP, PC3, DU145 LNCaP, PC3, DU145 PC3 |
LNCaP, PC3, DU145 grafted nude mice NA PC3-luc xenograft |
NA Synergism with docetaxel and cisplatin NA |
Huang et al. (2019) (Kim et al., 2010) (Zhang et al., 2015) |
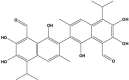 Gossypol (Polyphenolic) |
Gossypium hirsutum (Malvaceae) |
Blocked in vivo tumor proliferation by inducing DNA damage, p53, and apoptosis. Inhibited the expression of VEGF, Bcl-2, Bcl-xL, and tumor overload in vivo. Suppressed microvessel growth and sprouting in vitro and ex vivo. Inhibited p-Src (Tyr416), p-FAK (Tyr 397), p-Akt (S473), and p-ERK1/2 (Thr 202/Tyr 204) levels. Inhibited cell proliferation in vitro, and induced caspase-dependent apoptosis (Caspase3, -8), and blocks Bcl-2, PCNA, CD31 expression, and microvessel density in tumor tissue. Potentiated cell death in vitro and in vivo by apoptosis in combination with radiation; inhibited tumor angiogenesis. In combination, Sorafenib killed PCa cells more efficiently, involving apoptosis and autophagy in vitro and in vivo with reduced Bcl-2 level, Inhibited growth by apoptosis through mitochondrial pathway in PC3 cells and synergistically increased the anticancer activity of docetaxel both in vitro and in vivo. Blocked the expression of Bcl-xL while increasing the expression of Puma and Noxa. |
LAPC4, PC3, DU145 HUVEC, HMEC-1, PC3, DU145 PC3 PC3 PC3, DU145 PC3, DU145, LNCaP, WI38 |
DU145 xenograft in NOD/SCID mice PC3 xenograft and Sprague Dawley RAT PC3 xenograft PC3 grafted NCr-nu/nu nude mice PC3, DU145 xenograft PC3 grafted NCr-nu/nu nude |
NA NA NA Enhances the efficacy of radiotherapy Works synergistically with sorafenib Enhanced docetaxel efficacy in vitro and in vivo |
Volate et al. (2010) (Pang et al., 2011) (Zhang et al., 2010) (Xu et al., 2005) (Lian et al., 2012) (Meng et al., 2008) |
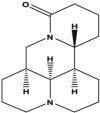 Matrine (Quinolizidine alkaloid) |
Sophora flavescens (Leguminosae) |
Blocked PCa cell growth, reversed EMT by activation of ER stress, and inhibition of unfolded protein response by inhibition of proteasomal chymotrypsin-like (CT-like) activity in the PCa cells in vitro and in vivo Blocked invasion through downregulating MMP2/9 and NF-κB signaling in vitro and in vivo |
DU145, PC3 DU145, PC3 |
DU145 xenograft DU145, PC3 xenografted nude mice |
NA NA |
Chang et al. (2018) (Huang et al., 2017) |
 Morelloflavone (Biflavonoid) |
Garcinia dulcis (Clusiaceae) | Prevented angiogenesis by reducing VEGF mediated microvessel formation and the RhoA and Rac1 GTPases. Blocked the phosphorylation and activation of Raf/MAPK/ERK pathway. Reduced tumor overload in vivo. | PC3, HUVEC | PC3 xenograft | NA | Pang et al. (2009) |
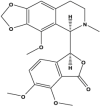 Noscapine (Benzylisoquinoline alkaloid) |
Papaver somniferum (Papaveraceae) |
Prophylactic feeding of Noscapine limited tumor growth and lymphatic metastasis. Enhanced the activity of paclitaxel and the Bax/Bcl-2 ratio. Reduced the mRNA levels of AR and PSA. |
PC3 LNCaP, PC3 |
PC3 xenografted athymic, Sim (NCr) nude mice NA |
NA Showed synergism with paclitaxel |
Barken et al. (2010) (Rabzia et al., 2017) |
 Silibinin (Flavolignan (Flavanoid unit linked to Phenyl Propane through an oxeran ring)) |
Silybum marianum (Asteraceae) | Hampered HIF-1α mediated signaling, angiogenesis, and lipogenesis in PCa cells. Reduced tumor load in vivo through inhibition of tumor vascularity; reduced the levels of HIF-1α, lipid, FASN, ACC, and NOX. . |
LNCaP, 22Rv1 | 22Rv1 xenograft | NA | (Deep et al., 2017) |
 Ursolic Acid (Pentacyclic triterpenoid) |
Cornus officinalis (Cornaceae) | Inhibited PI3K/Akt/mTOR pathway to induce apoptosis in vitro and in vivo | LNCaP, PC3 | LNCaP xenograft | NA | Meng et al. (2015) |
Abbreviations: Bax, Bcl2 associated protein X; Bcl2, B-cell leukemia/lymphoma 2; PI3K/Akt, phosphatidyl inositol-3-kinase/protein kinase B; EMT, epithelial to mesenchymal transition; ERK, extracellular signal regulatory protein kinase; NF-κB, nuclear factor kappa-light chain enhancer of activated B cells; CDKs, cyclin dependent kinases; AR, androgen receptor; PSA, prostate specific antigen; HIF-1α, hypoxia inducible factor-1 alpha; VEGF, vascular endothelial growth factor; STAT-3, signal transducer and activator of transcription 3; JNK, c-Jun-N-terminal kinase; PARP, poly (ADP-ribose) polymerase; AMPK, AMP-activated protein kinase; PTEN, phosphatase and tensin homolog; NKX3.1, NK3 Homeobox 1; PUMA, p53 upregulated modulator of apoptosis; NOXA, phorbol-12-myristate-13-acetate-induced protein 1; FOXO3a, forkhead box transcription factors 3a; TNF-α, tumor necrosis factor-α; MAPK, mitogen-activated protein kinase; Gn-Rh2, gonadotropin releasing hormone 2; CDKN1A, cyclin dependent kinase inhibitor 1A; Bak, Bcl-2 homologous antagonist/killer; Bad, Bcl-2 associated cell death; IκBα, I kappaB-alpha; PDK1, PIP3 dependent kinase 1; CRPC, castrate resistant prostate cancer; RhoA, ras homolog family member A; Rac1, ras-related C3 botulinum toxin substrate 1; PDEF, prostate epithelium-derived ETS transcription factor; Ki67, marker of proliferation Ki-67; PCNA, proliferating cell nuclear antigen; FOXM1, forkhead box M1; DHT, dihydrotestosterone; TRAMP, transgenic adenocarcinoma of mouse prostate; c-Src, phospho-proto-oncogene tyrosine-protein kinase c; phospho-focal adhesion kinase; Cdc2, cell division control 2; CD31, cluster of differentiation 31; FASN, fatty acid synthase; acetyl-CoA carboxylase; CNNM1, cyclin and CBS domain divalent metal cation transport mediator 1; IAP-1, inhibitor of apoptosis protein 1; XIAP, X-linked inhibitor of apoptosis protein; p-SMAD-2, phospho-mothers against decapentaplegic homolog 2; Raf, rapidly accelerated fibrosarcoma; VEGF-2, vascular endothelial growth factor-2.
