Abstract
Background
Intravenous fluid optimization is an essential component of managing patients in a critical care setting. A cumulative positive fluid balance is consistent with poor outcomes in patients admitted to the intensive care unit (ICU). The overall utility of net cumulative fluid balance as a surrogate for assessing fluid overload has been interrogated.
Materials and methods
This study was a prospective single-center observational study, which was done to correlate body weight changes with fluid balance in ICU patients and evaluate its impact on clinical outcomes. Inclusion criteria consisted of adult patients who were admitted to the critical care unit on specialized beds with integrated weighing scales between September 2017 and December 2018. The evaluation of the effect of changes in body weight on ICU survival was the primary objective of the study.
Results
We enrolled 105 patients in this study. The ICU mortality was 23.80% with non-survivors showing more weight gain than the survivors. Statistically significant weight gain was documented in the non-survivors on days 3 and 4 (1.9 vs 1.05; p = 0.0084 and 2.6 vs 1.6; p = 0.0030) of ICU admission. Non-survivors had greater cumulative positive fluid balance on fourth, fifth, and sixth days post-ICU admission when compared to survivors (3586 vs 1659 mL, p = 0.0322; 5418 vs 1255 mL, p = 0.0017; and 5430 vs 2305 mL p = 0.0264, respectively). In multivariate regression analysis, cumulative fluid balance did not correlate with days on mechanical ventilation or length of stay in ICU. Changes in body weight and cumulative fluid balance showed a good correlation.
Conclusion
In patients admitted to the ICU, weight gain on third and fourth days of admission is concordant with increased ICU mortality. Body weight changes were seen to correlate well with the cumulative fluid balance.
How to cite this article
Mishra RK, Pande A, Ramachandran R, Trikha A, Singh PM, Rewari V. Effect of Change in Body Weight on Clinical Outcomes in Critically Ill Patients. Indian J Crit Care Med 2021;25(9):1042–1048.
Keywords: Body weight, Fluid balance, Intensive care unit, Mortality
Introduction
Intravenous fluid administration is an essential component of hemodynamic resuscitation in ICU patients. As with any other intravenous drug, fluid therapy should also be prescribed judiciously and only when indicated. While optimizing volume status is mandatory for maintaining organ perfusion, excessive fluid transfusion may lead to adverse clinical outcomes. A net positive fluid balance is prognostic marker for intensive care unit (ICU) mortality.1 The deleterious effects of fluid overload are more likely during critical illness due to altered vascular permeability.2 This may result in impairment of renal function, deteriorating gas exchange, and impaired wound healing, all of which may potentially worsen the proinflammatory state.3 For patients in sepsis and septic shock, an independent marker of mortality is a positive cumulative fluid balance (CFB).4,5 Excessive fluid administration leads to expansion of interstitial space volume, which results in organ dysfunction. It leads to increased renal congestion and elevated renal subcapsular pressures, thereby resulting in acute kidney injury.6 A positive CFB is a consistent risk factor leading to development of intra-abdominal hypertension.7
Various techniques have been used in an attempt to quantify fluid balance.8 The most used technique is the daily charting of the fluid balance. Hourly recording of fluid intake and output is a routine part of nursing care in most ICUs. However, these recordings are susceptible to errors,9 more so when larger calculations are necessary. Furthermore, fluids administered to maintain catheter patency and fluid flushes after drug administration—the “fluid creep”—are often not recorded.10 The charting of daily fluid balance also does not account for the insensible fluid losses (IFLs). To overcome these fallacies, alternative techniques like daily body weight measurement and total body plethysmography have been suggested to assess fluid balance.11 Commercially available electronic beds can be used for daily assessment of patient's weight, which has been used to measure fluid balance with varying results.12,13 Body weight changes in ICU patients are attributed to a myriad of factors like nature of illness, fluid balance, and nutritional status. There is evidence to suggest that increase in body weight during the course of critical illness is associated with increased ventilatory days as well as ICU stay.14 To date, there is insufficient evidence to suggest the superiority of one technique of assessment of fluid status over the other in terms of patient-centered outcomes like mortality. Hence, to demonstrate the effect of changes in body weight on survival in a multidisciplinary critical care unit, we designed this prospective cohort study.
Materials and Methods
This single-center observational study was undertaken prospectively in a population of mixed medical and surgical patients in a critical care unit of a tertiary health center. After obtaining adequate approval from the Institutional Ethics Committee (IECPG-178/23.08.2017 with effect from September 7, 2017), patients were enrolled in this study after written informed consent from patients’ next of kin.
Study Population
All ICU patients who were admitted from September 2017 to December 2018 were screened for eligibility. Inclusion criteria consisted of all patients who were aged 18 years and above with an expected ICU length of stay of more than 48 hours. If the patients weighed more than 150 kg or if the weighing bed was not tared prior to admission, then the patients were excluded from the study.
Procedure
Body weight measurements in this study were done with ICU beds, which have an integrated weighing scale (MultiCare, Linet, and Hill-Rom, Batesville, Indiana). The weighing bed was calibrated prior to receiving the patient on it. The tare procedure was performed by the nurses with two bedsheets and one pillow only. Body weight was recorded on admission and daily at 7 a.m. after the routine patient care was completed. At all times, it was ensured that no extra item was placed on the bed during the process of measurement. All ICU nurses were trained in the correct procedure of weighing the patient before the start of the study.
The total intake was calculated by adding the hourly entry for inputs of all fluids including blood and blood products, intravenous drugs, and nutrition, while the total output was calculated by adding the urine output, drain contents, and estimated fecal volume. The fluid balance (FB) was calculated at 7 a.m. daily using simple mathematics of total fluid intake minus the total output.
IFL was calculated according to a formula suggested by Peter Cox.15 IFL (mL) = 800 + 20% × 800 × (maximum temperature − 37°C). This value was divided by 2 in an intubated patient.
Data Collection
All demographics like height, weight, age, duration of ICU stay, and duration of mechanical ventilatory support were recorded. Acute physiological scores viz. acute physiology and chronic health evaluation (APACHE) score and sequential organ failure score (SOFA) were assessed for all patients at baseline as indicators for severity of illness. Daily records of fluid intake including enteral feed, parenteral nutrition, iv fluids, and intravenous medication and fluid output including urine, stool, drain output, NG aspirate, and blood loss were maintained. Fluid balance charts were maintained and locked at 7 a.m. daily. Laboratory values like hemoglobin, blood urea, serum creatinine, and albumin were also recorded. Requirement of renal support and hemodynamic support in terms of vasopressor requirement was noted as well.
The endpoint of follow-up was either death or ICU discharge. The primary objective of this study was to assess the effect of body weight changes on ICU survival in a study population. The secondary outcomes included correlation of cumulative fluid balance (CFB) and body weight (BW); and the effects of CFB on ICU survival. The effects of changes in CFB and BW on length of ICU stay, duration of mechanical ventilation and vasopressor therapy, and in-hospital mortality were also evaluated.
Statistical Analysis
Stata 14.0 software was used for all statistical calculations and data analysis. Frequency and percentage interpretation was done for all categorical data. Quantitative data were expressed as mean ± standard deviation and median (minimum, maximum) for data following normal and skewed distribution. Categorical data were compared by chi-square or Fisher's exact test. All comparisons and qualitative data were assessed by independent t-test and Mann–Whitney rank sum test for normally distributed and skewed data, respectively. Regression analysis is carried out to estimate changes in body weight with fluid balance adjusting for age, SOFA, and APACHE II. Pearson/Spearman correlation coefficient was used to find the relationship between quantitative variables. Two-tailed p values <0.05 were taken as a point of statistical significance. Agreement between weight change and fluid balance was done using a Bland–Altman plot.
Results
Enrolled population consisted of 105 patients in the study. Table 1 represents the baseline data of survivors and non-survivors. The weight (mean ± SD) on admission was 45.8 ± 21.27 kg, and the height (mean ± SD) was 160 ± 10.73 cm. As expected, SOFA (median 10 for non-survivors vs 5 for survivors) and APACHE II (median 15 for non-survivors vs 11 for survivors) scores were significantly more in non-survivors. Moreover 76.19% of patients survived till discharge, of which two patients died in hospital after ICU discharge. Mortality in ICU was 23.80%, and mortality rate in hospital was 25.71%. Acute kidney injury developed in 31.43% of all patients. Majority of the recruited patients (76.19%) required mechanical ventilation, and 51.43% of patients required vasopressors. Also, non-survivors had prolonged duration of stay in ICU, length of mechanical ventilatory support and higher dose and duration of vasopressors during their ICU course. Prolonged length of mechanical ventilation was observed in non-survivors (median of 104 vs 40 hours for the survivors). All patients who never required ventilatory support during their course of stay in ICU were discharged. Requirement of vasopressors was high among the non-survivors with a median (range) duration of 25 (3–122) hours among the survivors vs 96 (18–460) among the non-survivors. Mean length of stay in ICU (mean ± SD) was 138.62 ± 130.57 hours, and length of mechanical ventilation (mean ± SD) was 101.3 ± 150.47 hours.
Table 1.
Baseline characteristics of survivors and non-survivors
| Variable | Survivors (n = 80) | Non-survivors (n = 25) | p value |
|---|---|---|---|
| Age (years) | 43 (18–86) | 56 (18–85) | 0.072 |
| Height (cm) | 159 (126–178) | 165 (144–178) | 0.043* |
| Weight (kg) | 60.95 (36–93.5) | 62.3 (36.4–111.6) | 0.810 |
| BMI (kg/m2) | 24.81 (13.95–36.37) | 24.41 (14.5–38.9) | 0.441 |
| LOS (ICU) (hrs) | 95 (68–840) | 122 (76–1008) | 0.0088* |
| APACHE II | 11 (4–30) | 15 (8–32) | 0.0009* |
| SOFA | 5 (2–14) | 10 (5–15) | 0.001* |
| Hb (gm/dL) | 10.5 (4.3–22.7) | 9.3 (6.1–16.8) | 0.568 |
| Bl. urea (mg/dL) | 33 (11–443) | 62 (17–259) | 0.0209* |
| Sr. creatinine (mg/dL) | 0.9 (0.2–15.9) | 1.7 (0.5–8.9) | 0.0032* |
| Albumin (gm/dL) | 3.1 (1.1–4.7) | 2.9 (1.9–6.4) | 0.2766 |
| LMV (hrs) | 40 (2–786) | 104 (20–1008) | 0.0001* |
| Noradrenaline (mg) | 6 (0.05–4.8) | 94.2 (4.8–240) | 0.001* |
| Vaso (units) | 15 (4–38.4) | 124 (36–460) | 0.0024* |
| Adr (mg) | 11.75 (11.5–12) | 17 (1.2–100) | 0.003* |
| Duration of vaso (hrs) | 25 (3–122) | 96 (18–460) | 0.0001* |
| No. of RRT | 1 (1–1.4) | 1 (1–5) | 0.59 |
Data are presented as median (min;max); BMI, body mass index; LOS (ICU), length of stay (intensive care unit); Hb, hemoglobin; Bl. urea, blood urea; Sr. creatinine, serum creatinine; LMV, length of mechanical ventilation; Vaso, vasopressin; Adr, adrenaline; Duration of vaso, duration of vasopressors in ICU; No. of RRT, No. of renal replacement therapies;
Statistically significant
Outcomes
Weight Change
Assessment of the effect of changes in body weight on survival was the primary outcome of the study. Non-survivors gained more weight than survivors till day 9 of ICU stay, after which survivors gained greater weight. The weight gain in non-survivors was significantly greater than survivors in days 3 and 4 of ICU admission (1.9 vs 1.05 kg; p = 0.0084 and 2.6 vs 1.6 kg; p = 0.030, respectively). The time trend of weight fluctuation among those who survived and who did not is shown in Table 2.
Table 2.
Comparison of weight change of survivors and non-survivors
| Weight change from D1 | Survivors (n = 80) | Non-survivors (n = 25) | p value |
|---|---|---|---|
| Day 2 | 0.72 (−1.9 to 4.5) | 0.8 (−3.7 to 4.3) | 0.9579 |
| Day 3 | 1.05 (−3.7 to 6.7) | 1.9 (−4.3 to 5.6) | 0.0084* |
| Day 4 | 1.6 (−5.84 to 7) | 2.6 (−1.9 to 6.2) | 0.0030* |
| Day 5 | 1.8 (−9.95 to 6.3) | 3.375 (−2.5 to 7.9) | 0.0620 |
| Day 6 | 2.2 (−9.65 to 5.6) | 3.5 (−1.4 to 8.6) | 0.1277 |
| Day 7 | 1.8 (−4.7 to 5.6) | 3.1 (−1.8 to 7) | 0.1488 |
| Day 8 | 2.3 (−5.7 to 5.2) | 2.6 (−2.05 to 6.9) | 0.4378 |
| Day 9 | 2.35 (−1.1 to 6.9) | 2.65 (−1.4 to 6.95) | 0.8587 |
| Day 10 | 1.9 (0.2 to 8.4) | 1.9 (−0.8 to 7.4) | 0.6249 |
| Day 11 | 3.35 (−0.7 to 8.5) | 2.4 (−1.2 to 7.8) | 0.7150 |
| Day 12 | 4.2 (−3.7 to 9.4) | 1.6 (−3.2 to 8.2) | 0.6242 |
| Day 13 | 4.65 (0.9 to 8.9) | 1 (−2.1 to 9.4) | 0.7237 |
| Day 14 | 3.65 (2.1 to 8.5) | 0.7 (−2.6 to 11.3) | 0.4795 |
| Day 15 | 3.25 (0.5 to 5.4) | 3.1 (−2.8 to 11) | 0.7237 |
Data are expressed as median (range) weight change;
Statistically significant difference; All numerics expressed in kilograms
Fluid Balance
Non-survivors had significantly higher positive cumulative fluid balance on fourth, fifth, and sixth days compared to survivors (3586 vs 1659 mL, p = 0.0322; 5418 vs 1255 mL, p = 0.0017; and 5430 vs 2305 mL, p = 0.0264, respectively). Although on most of the ICU days, patients who did not survive had higher cumulative fluid balance than those who survived, it was not statistically significant (Table 3).
Table 3.
Comparison of fluid balance of survivors and non-survivors
| FB | Survivors | Non-survivors | p value |
|---|---|---|---|
| Day 1 | 14 (−2895 to 4553) | 449 (−1650 to 13954) | 0.1805 |
| Day 2 | 635 (−1890 to 7400) | 1503 (−1668 to 16100) | 0.1709 |
| Day 3 | 1146 (−3645 to 9350) | 2616 (−1920 to 27730) | 0.0580 |
| Day 4 | 1659 (−3289 to 12084) | 3586 (−2266 to 22400) | 0.0322* |
| Day 5 | 1255 (−3904 to 12952) | 5418 (400 to 23895) | 0.0017* |
| Day 6 | 2305 (−3275 to 11407) | 5430 (−415 to 14640) | 0.0264* |
| Day 7 | 1281 (−4966 to 12987) | 5865 (−1075 to 14503) | 0.0825 |
| Day 8 | 3117 (−4866 to 13867) | 7605 (−1305 to 15088) | 0.0881 |
| Day 9 | 5775 (−4756 to 14437) | 10514 (1402 to 17400) | 0.20503 |
| Day 10 | 8159 (4390 to 14512) | 2697 (2452 to 17734) | 0.4561 |
| Day 11 | 8604 (4506 to 13362) | 4532 (636 to 17734) | 0.7237 |
| Day 12 | 9162 (5990 to 11370) | 7825 (739 to 18516) | 0.4795 |
| Day 13 | 8632 (6735 to 11600) | 5014 (573 to 20606) | 0.4795 |
| Day 14 | 8220 (6980 to 11650) | 5692 (879 to 23300) | 0.4795 |
| Day 15 | 9310 (7913 to 11675) | 1445 (1445 to 1445) | 0.1797 |
Data are expressed as median (range); FB, fluid balance;
Statistically significant; Fluid balance expressed in milliliters
Correlation between Changes in Body Weight and Cumulative Fluid Balance
Correlation analysis was done using Pearson correlation tests between daily changes in body weight measured on bed scale and daily CFB charts taking into consideration the insensible fluid losses (Fig. 1). The correlation was found to be significant on day 2 through day 5 and on days 10 and 14 (Pearson correlation coefficient r on day 2 = 0.423, p = 0.001; r on day 3 = 0.309, p = 0.001; r on day 4 = 0.352, p = 0.003; r on day 5 = 0.409, p = 0.003; r on day 10 = 0.77, p = 0.023; and r on day 14 = 0.818, p = 0.047). Higher correlation toward the end of ICU stay may not be considered because of the lesser number of patients and readings toward the end of second week of ICU stay.
Fig. 1.
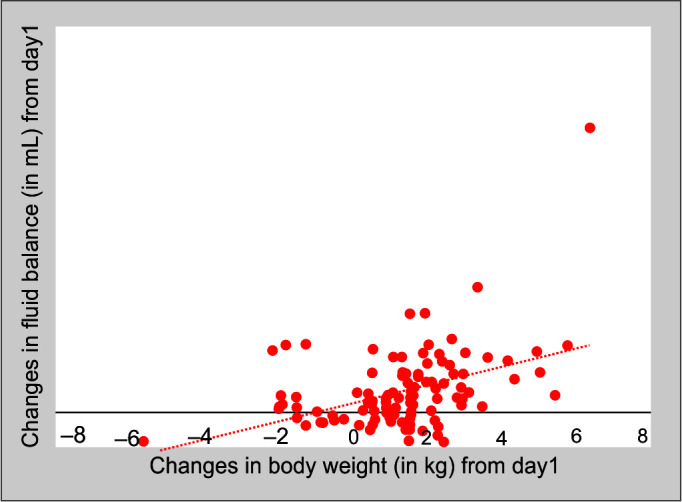
Correlation between weight change and fluid balance
Both univariate linear regression analysis and multivariate linear regression analysis of body weight changes and fluid balance with duration of mechanical ventilation in the initial 6 days of ICU stay revealed no correlation (Table 4).
Table 4.
Univariate linear regression analysis for duration of mechanical ventilation
| Variables | ß | Standard error | p value | 95% confidence interval |
|---|---|---|---|---|
| Age | 0.410 | 1.64 | 0.086 | −3.02 to 3.84 |
| SOFA | 31.252 | 18.64 | 0.109 | −7.6 to 70.15 |
| APACHE II | −5.601 | 9.60 | 0.566 | −2.56 to 14.43 |
| Wt Ch day 2 | −21.009 | 39.48 | 0.601 | −103.3 to 61.35 |
| Wt Ch day 3 | 9.634 | 34.12 | 0.781 | −61.55 to 80.82 |
| Wt Ch day 4 | −32.484 | 44.17 | 0.471 | −124.62 to 59.65 |
| Wt Ch day 5 | 27.83 | 27.83 | 0.501 | −56.88 to 112.55 |
| Wt Ch day 6 | −9.98 | 30.55 | 0.747 | −73.72 to 53.74 |
| FB day 2 | 0.063 | 0.128 | 0.602 | −2.03 to 0.332 |
| FB day 3 | 0.006 | 0.125 | 0.958 | −2.544 to 0.267 |
| FB day 4 | 0.020 | 0.061 | 0.739 | −0.510 to 0.108 |
| FB day 5 | 0.121 | 0.177 | 0.501 | −2.98 to 0.445 |
| FB day 6 | 0.064 | 0.178 | 0.685 | −0.298 to 0.445 |
SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation score; Wt Ch, weight change; FB, fluid balance
Univariate regression analysis between weight change and fluid balance and ICU length of stay revealed positive fluid balance on days 2 and 3 was associated with prolonged length of ICU stay (day 3, ß = 0.033 p = 0.044, 95% CI −0.05 to 0.125; day 4 ß = 0.012, p = 0.021, 95% CI −0.05 to 0.033). There was no significant correlation between weight change and ICU duration of stay (Table 5). Multivariate regression analysis of weight change and fluid balance with ICU duration of stay did not reveal any correlation.
Table 5.
Univariate linear regression analysis for length of ICU stay
| Variables | ß | Standard error | p value | 95% confidence interval |
|---|---|---|---|---|
| Age | −1.402 | 0.581 | 0.812 | −1.35 to 1.07 |
| SOFA | 0.592 | 7.02 | 0.934 | −14.10 to 15.285 |
| APACHE II | −0.703 | 3.414 | 0.839 | −7.85 to 6.44 |
| Wt Ch day 2 | 9.461 | 14.01 | 0.508 | −19.87 to 38.80 |
| Wt Ch day 3 | 1.617 | 12.05 | 0.895 | −23.61 to 26.84 |
| Wt Ch day 4 | −16.199 | 15.78 | 0.318 | −49.22 to 16.830 |
| Wt Ch day 5 | 7.70 | 14.48 | 0.601 | −22.61 to 38.01 |
| Wt Ch day 6 | −0.250 | 10.79 | 0.982 | −22.85 to 22.35 |
| FB day 2 | 0.042 | 0.045 | 0.748 | −0.13 to 0.05 |
| FB day 3 | 0.033 | 0.044 | 0.044* | −0.05 to 0.125 |
| FB day 4 | −0.012 | 0.021 | 0.021* | −0.05 to 0.033 |
| FB day 5 | −0.015 | 0.063 | 0.063 | −0.148 to 0.117 |
| FB day 6 | −0.017 | 0.063 | 0.063 | −0.14 to 0.114 |
SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation score; Wt Ch, weight change; FB, fluid balance;
Statistically significant
Positive fluid balance correlated with a longer duration of vasopressor therapy on days 4, 5, 6, and 7 (day 4 r = 0.523, p = 0.015; day 5 r = 0.654, p = 0.036; day 6 r = 0.654, p = 0.029; and day 7 r = 0.678, p = 0.031). However, correlation between changes in body weight and duration of vasopressor therapy was significant only on day 11 (r = 0.834, p = 0.039).
Agreement between Fluid Balance and Body Weight
On day 3, the mean difference was 0.487 kg and the limits of agreement were between −5.95 and 4.981 kg. For day 5, the mean difference was 0.825 kg and limits of agreement were −8.33 and 6.679 kg. Limits of agreement were wide, but good agreement was observed between the changes in body weight and cumulative fluid balance (Figs 2 and 3).
Fig. 2.
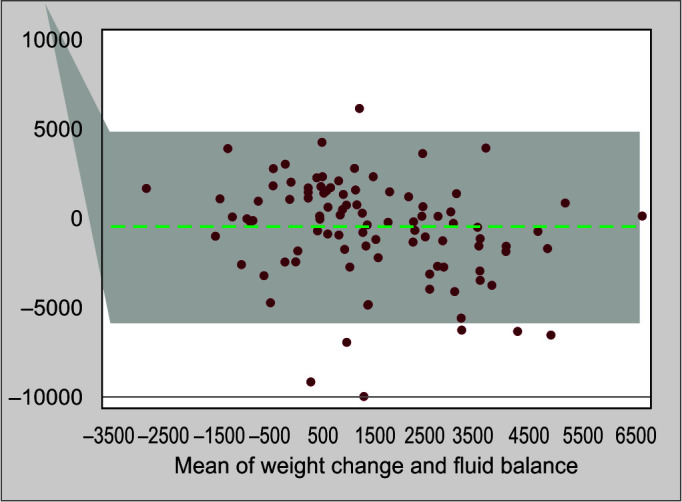
Bland–Altman plot depicting the agreement between fluid balance and weight change on day 3
Fig. 3.
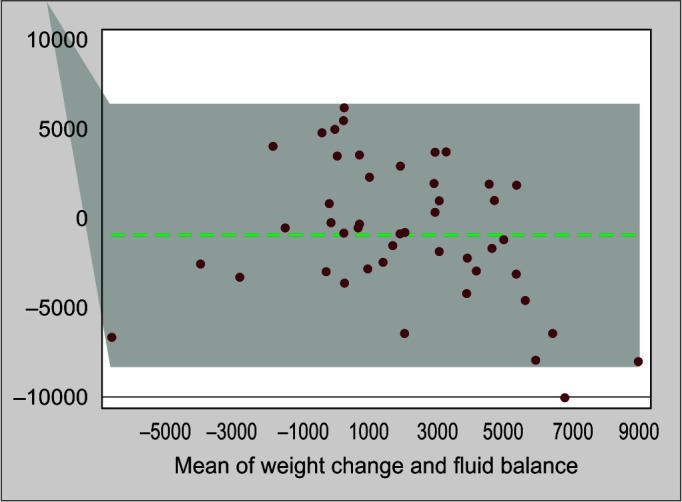
Bland–Altman plot showing the agreement between fluid balance and weight change on day 5
Discussion
This study demonstrates that weight gain from baseline body weight at the time of admission to ICU was concordant with increased mortality on third and fourth days of ICU admission. The non-survivors in the study had higher weight gain when compared to survivors, on most of the days in the ICU. Correlation between weight gain and fluid balance was significant from day 2 to day 5 and subsequently on days 10 and 14. However, changes in body weight and length of ICU stay and mechanical ventilatory support did not reveal any association. Furthermore, a good correlation was seen between body weight changes and fluid balance.
Fluid overload occurs commonly during the course of critical illness.16 It is intuitive to have easy and reproducible techniques to monitor fluid status, which may be utilized to guide further therapy and simultaneously avoid complications.17 Fluid balance charts or weighing the patients daily may be used toward this purpose. It is yet unclear as to which technique is more reliable and accurate in critically ill patients.18 Calculation of fluid balance is often imprecise and prone to multiple errors.19
Weighing bed-bound patients using conventional weighing scales was an arduous task till recent past. With the introduction of ICU beds with integrated weighing scales, body weight may be used as a surrogate marker of fluid balance.20 Although utilized in previous studies,21 there was poor adherence to a protocol of daily weighing of the patients. In their systematic review, Davies et al. found that inability to measure the body weight daily was the major hindrance to the utility of this technique to be an appropriate marker of fluid balance.22 In our study, all patients admitted for more than 48 hours were weighed daily at 7 a.m., which was feasible due to the availability of automated weighing beds.
Body weight fluctuates during the course of critical illness due to multiple factors like fluid balance, insensible fluid loss, varied nutritional support, and different phases of catabolism.14 Although weight fluctuations are observed widely in clinical practice, the effect of this change on outcomes of ICU patients is unclear. Some studies have demonstrated that the correlation between changes in body weight and CFB was poor and fluid balance charts were not at par in predicting sequential body weight changes.9,12 We found that weight gain on third and fourth days of ICU admission was associated with higher mortality. This may be explained by the fact that aggressive resuscitation is undertaken in the first 48 hours of admission in patients who have profound hemodynamic perturbations. The patients who respond well to this management usually achieve an adequate urine output. The patients in whom optimal tissue perfusion cannot be attained despite adequate resuscitation may remain oliguric and thereby have a higher positive fluid balance. In fact, volume-related weight gain may be an independent indicator of requirement of renal support in ICU patients.23 Furthermore, after day 9 of ICU admission, survivors had higher weight gain, possibly reflective of an anabolic state.
Our findings are similar to those observed by You et al. who found that ICU patients with higher cumulative positive fluid balance between days 2 and 7 of admission to ICU had a higher mortality.14 However, unlike their findings, we could not demonstrate any concordance between body weight changes and length of ICU stay or mechanical ventilation. Similarly, Wiedemann et al. evaluated 1,000 patients receiving conservative vs liberal fluid management strategy.23 They found that patients with conservative fluid strategy fared better in terms of reduced days on ventilatory support, oxygenation index, and decreased duration of ICU stay without worsening shock or increasing requirement of renal replacement therapy. This difference may be explained partly by the fact that weaning and extubation were at the discretion of the treating physician in our ICU and therefore were not standardized.
Similar to our study, Chittawatanarat et al. also observed higher mortality in surgical critically ill patients with acute body weight change of greater than 5% occurring over first week of critical illness.24 However, they defined a cutoff value of 5% arbitrarily. To date, there is insufficient evidence to consider a standard cutoff value as reference for significant weight gain. Although our results were similar to those observed by Acheampong et al.,5 we also included insensible fluid losses in our study by a predictive equation, which was not accounted for in their study.
The strengths of our study include giving due consideration to insensible fluid losses while estimating the fluid balance. This study did a comprehensive analysis of the various outcomes in a heterogeneous ICU population using integrated bed scales in all the patients. Secondly, there was strict adherence to the weighing protocol, which was important to get reliable results.
There were some limitations in our study. Firstly, as it was a single-center study, its external validity remains limited. Secondly, this was an observational study done on limited number of patients and further studies done on a larger number of patients may help in formulating more accurate associations. Thirdly, although an association was found between weight change and mortality, it does not translate into a cause–effect relationship as multiple factors may be responsible for influencing the patient's weight and fluid requirements. Next, the inaccuracy of calculations of fluid balance could have been mitigated by maintaining computerized records with automated calculations. Furthermore, the use of diuretics may impact fluid balances and body weight, which was not taken into consideration. Also, catabolic state in sepsis has a great bearing on the weight of the patients, which is difficult to estimate. Lastly, some other techniques like bioelectrical impedance analysis could also have been used to detect if the change in weight could be attributed due to fluid accumulation alone.25
Conclusion
In patients admitted to the ICU, weight gain on third and fourth days of admission is associated with increased ICU mortality. Body weight changes were seen to correlate well with cumulative fluid balance and hence may be used as a surrogate marker for the same.
Footnotes
Source of support: Nil
Conflict of interest: None
Orcid
Rajesh K Mishra https://orcid.org/0000-0002-4400-1625
Aparna Pande https://orcid.org/0000-0001-6004-1099
Rashmi Ramachandran https://orcid.org/0000-0001-6083-7513
Anjan Trikha https://orcid.org/0000-0002-6001-8486
Preet M Singh https://orcid.org/0000-0001-7642-529X
Vimi Rewari https://orcid.org/0000-0001-9800-1367
References
- 1.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Duan C-Y, Zhang J, Wu H-L, Li T, Liu L-M. Regulatory mechanisms, prophylaxis and treatment of vascular leakage following severe trauma and shock. Military Med Res. 2017;4:11. doi: 10.1186/s40779-017-0117-6. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malbrain MLNG, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361–380. doi: 10.5603/AIT.2014.0060. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S, et al. Higher fluid balance increases the risk of death from sepsis: results from a large international audit. Crit Care Med. 2017;45(3):386–394. doi: 10.1097/CCM.0000000000002189. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Acheampong A, Vincent J-L. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015;19(1):251. doi: 10.1186/s13054-015-0970-1. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patil VP, Salunke BG. Fluid overload and acute kidney injury. Indian J Crit Care Med. 2020;24(Suppl. 3):S94–S97. doi: 10.5005/jp-journals-10071-23401. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reintam Blaser A, Regli A, De Keulenaer B, Kimball EJ, Starkopf L, Davis WA, et al. Incidence, risk factors, and outcomes of intra-abdominal hypertension in critically ill patients – a prospective multicenter study (IROI Study). Crit Care Med. 2019;47(4):535–542. doi: 10.1097/CCM.0000000000003623. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basso F, Berdin G, Virzì GM, Mason G, Piccinni P, Day S, et al. Fluid management in the intensive care unit: bioelectrical impedance vector analysis as a tool to assess hydration status and optimal fluid balance in critically ill patients. Blood Purif. 2013;36(3–4):192–199. doi: 10.1159/000356366. DOI: [DOI] [PubMed] [Google Scholar]
- 9.Perren A, Markmann M, Merlani G, Marone C, Merlani P. Fluid balance in critically ill patients. Should we really rely on it? Minerva Anestesiol. 2011;77(8):802–811. PMID: [PubMed] [Google Scholar]
- 10.Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44(4):409–417. doi: 10.1007/s00134-018-5147-3. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies H, Leslie G, Jacob E, Morgan D. Estimation of body fluid status by fluid balance and body weight in critically ill adult patients: a systematic review. Worldviews Evid Based Nurs. 2019;16(6):470–477. doi: 10.1111/wvn.12394. DOI: [DOI] [PubMed] [Google Scholar]
- 12.Schneider AG, Baldwin I, Freitag E, Glassford N, Bellomo R. Estimation of fluid status changes in critically ill patients: fluid balance chart or electronic bed weight? J Crit Care. 2012;27(6):745.e7–e12. doi: 10.1016/j.jcrc.2011.12.017. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Köster M, Dennhardt S, Jüttner F, Hopf H-B. Cumulative changes in weight but not fluid volume balances reflect fluid accumulation in ICU patients. Acta Anaesthesiol Scand. 2017;61(2):205–215. doi: 10.1111/aas.12840. DOI: [DOI] [PubMed] [Google Scholar]
- 14.You J-W, Lee SJ, Kim YE, Cho YJ, Jeong YY, Kim HC, et al. Association between weight change and clinical outcomes in critically ill patients. J Crit Care. 2013;28(6):923–927. doi: 10.1016/j.jcrc.2013.07.055. DOI: [DOI] [PubMed] [Google Scholar]
- 15.Cox P. Insensible water loss and its assessment in adult patients: a review. Acta Anaesthesiol Scand. 1987;31(8):771–776. doi: 10.1111/j.1399-6576.1987.tb02662.x. DOI: [DOI] [PubMed] [Google Scholar]
- 16.Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients – a systematic review and meta-analysis of observational studies. Crit Care Med. 2020;48(12):1862–1870. doi: 10.1097/CCM.0000000000004617. DOI: [DOI] [PubMed] [Google Scholar]
- 17.Claure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 2016;17(1):109. doi: 10.1186/s12882-016-0323-6. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peacock WF, Soto KM. Current techniques of fluid status assessment. Contrib Nephrol. 2010;164:128–142. doi: 10.1159/000313726. DOI: [DOI] [PubMed] [Google Scholar]
- 19.Manoj R, Kumarasami R, Joseph J, George B, Sivaprakasam M. 2019 IEEE international symposium on medical measurements and applications (MeMeA). Istanbul, Turkey: IEEE; 2019. Continuous weight monitoring system for ICU beds using air-filled mattresses/pads: a proof of concept. pp. 1–5. p. [Google Scholar]
- 20.Tolstrup J, Brandstrup B. Clinical assessment of fluid balance is incomplete for colorectal surgical patients. Scand J Surg. 2015;104(3):161–168. doi: 10.1177/1457496914543978. DOI: [DOI] [PubMed] [Google Scholar]
- 21.Eastwood GM. Evaluating the reliability of recorded fluid balance to approximate body weight change in patients undergoing cardiac surgery. Heart Lung J Crit Care. 2006;35(1):27–33. doi: 10.1016/j.hrtlng.2005.06.001. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Davies H, Leslie GD, Morgan D, Dobb GJ. A comparison of compliance in the estimation of body fluid status using daily fluid balance charting and body weight changes during continuous renal replacement therapy. Aust Crit Care. 2019;32(2):83–89. doi: 10.1016/j.aucc.2017.12.090. DOI: [DOI] [PubMed] [Google Scholar]
- 23.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. DOI: [DOI] [PubMed] [Google Scholar]
- 24.Chittawatanarat K, Pichaiya T, Chandacham K, Jirapongchareonlap T, Chotirosniramit N. Fluid accumulation threshold measured by acute body weight change after admission in general surgical intensive care units: how much should be concerning? Ther Clin Risk Manag. 2015;11:1097–1106. doi: 10.2147/TCRM.S86409. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myatchin I, Abraham P, Malbrain MLNG. Bio-electrical impedance analysis in critically ill patients: are we ready for prime time? J Clin Monit Comput. 2020;34(3):401–410. doi: 10.1007/s10877-019-00439-0. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]


