Abstract
The endocannabinoid system is chiefly recognized as a homeostatic regulator of synaptic neurotransmission, primarily through the modulation of presynaptic CB1 cannabinoid neurons. Accordingly, the use of plant-derived cannabinoids received significant attention recently given the broad spectrum of physiological and pathobiological processes the endocannabinoid system is involved in. Nevertheless, a parallel line of research from a number of developmental biology groups has uncovered fundamental, evolutionarily conserved, and molecularly unique processes that endocannabinoids drive during development of the central nervous system. This lecture transcript is a concise summary of nearly 20 years of research on endocannabinoid-gated mechanisms of neurogenic specification events, which particularly define the numbers, placement, and connectivity of cortical neurons. A summary of both CB1 and alternative cannabinoid receptor contributions to neural differentiation is also discussed. Besides, insights are given into how phytocannabinoids can bypass physiologically timed and pivoted endocannabinoid action to inflict developmental errors that can significantly compromise the adaptive and computational ability of neurocircuits. By discussing specific subcellular targets of phytocannabinoid action and inferring errant glia versus neuron fate decisions and communication, a cellular basis is outlined for lifelong psychiatric phenotypes in offspring that associate with maternal cannabis seeking during pregnancy.
Keywords: axon guidance, cell fate, corticogenesis, neurogenesis, synapse
Introduction by Daniele Piomelli
On September 16, 2020, Cannabis and Cannabinoid Research and ICAL (Impact of Cannabinoids Across the Lifespan), a center of excellence funded by the National Institute on Drug Abuse, organized an international virtual symposium dedicated to Cannabis in a changing brain—the evolving impact of cannabinoids across the lifespan. A panel of distinguished speakers, including Drs. Tibor Harkany, Daniela Parolaro, Tiziana Rubino, and Andreas Zimmer, discussed the roles of endocannabinoid signals in the prenatal and postnatal development of the brain, and how such development alters the response to cannabinoid agents. What follows is an edited transcription of Dr. Harkany's lecture, entitled “Physiological rules of endocannabinoid action during fetal and neonatal brain development.”
Lecture on Physiological Rules of Endocannabinoid Action During Fetal and Neonatal Brain Development
In this lecture today, we would like to share with you highlights of the research conducted in our laboratory on the functions served by the endocannabinoid system in brain development as well as on the impact of cannabis's intoxicating constituent, Δ9-tetrahydrocannabinol (THC), on this process. To begin with, we would like to highlight two key concepts that are relevant for both human and mouse brain development (Fig. 1A). First, neurogenesis and neuronal specification occur before the progeny reaches the specific brain areas, which they will populate. And, second, neuronal migration1 is followed by a series of important events, which are the development of glial cells, the process of myelination and the formation of connectivity across brain structures with the creation of axons and synapses.2 These processes do not stop by termed birth and indeed last well into adolescence or even later; some researchers would argue even up to 25 years of age.
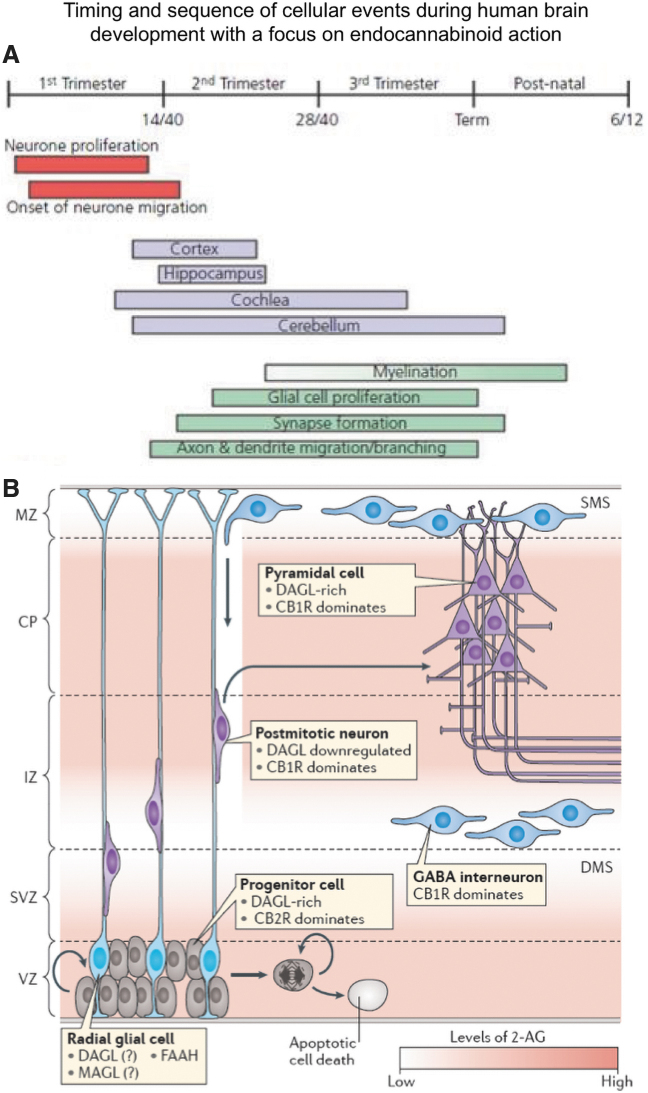
FIG. 1.
The sequence of corticogenesis and endocannabinoid contributions therein. (A) Pre- and postnatal milestones of neuro- and gliogenesis during human brain development. (B) Endocannabinoid contributions to corticogenesis, including (1) stem cell proliferation, (2) stem cell survival, (3) neuron versus glia fate decision, (4) neuronal migration, (5) synaptogenesis and axonal growth. This is achieved through the likely existence of endocannabinoid gradients (graded shading) and a mixture of cell autonomous/intercellular signaling events. CP, cortical plate; dms/sms, deep/superficial migratory streams; IZ, intermediate zone; MZ; marginal zone; SVZ, subventricular zone; VZ, ventricular zone. Reproduced with permission from Maccarrone et al.5
The question is, what are the guiding forces behind these developmental rules and these developmental processes? During the past 40 years, there has been a tremendous amount of research done on the different types of signaling systems that shape the complexity of the mammalian nervous system.3,4 Research over the past 15 years has shown that endocannabinoids such as anandamide and 2-arachidonoyl-glycerol (2-AG) represent one such system.5 The main message of this lecture is that endocannabinoids influence brain development at multiple levels (Fig. 1B).
The first is the stem cell niche: the number and the division rate of stem cells is regulated by endocannabinoid signals. The second is the decision of what kind of neural cell progeny is produced, neurons or glia, which is also under endocannabinoid control. Once the progeny is generated, the third level at which endocannabinoids influence brain development is the migration of the neural cell progeny to their final positions in the cerebral cortex and other brain areas, a long-range travel over a distance of millimeters or centimeters.6,7 And after these cells have arrived at their final location, endocannabinoid molecules can affect how they build active and integrated neuronal networks.8 Both type-1 and type-2 cannabinoid receptors (CB1 and CB2) may be involved in these diverse processes,9 but today focus will be on the role of endocannabinoid signaling at CB1 receptors in the control of migration6 and neuronal network formation.10
Next, we showed the localization of CB1 receptors in the midterm human fetus,7 after ∼20 weeks of pregnancy. Similar to rodent models, the hippocampus and the parahippocampal gyrus are particularly rich in CB1 receptors. Moreover, cells that have reached the cortical plate and home in there, as well as those sitting in progenitor zones, show various levels of CB1 receptor expression. This is relevant from a public health perspective because a relatively high percentage of women are exposed to cannabis during pregnancy,11,12 which raises the question of whether THC might engender modifications that might be detrimental for brain development.5 To address this question, it is essential to dissect the role of the endocannabinoid system in the developing brain. I will outline model studies, particularly in mice, which justify our conclusions on the relevance and importance of this signaling complex.
When we look at the developing mouse brain, what is especially significant is that CB1 receptors are highly expressed in cortical areas, for example, in corticofugal axons13 (Fig. 2A). Another important finding is that CB1 receptors are expressed in both excitatory pyramidal cells7 and inhibitory interneurons6,10 and are partitioned toward their developing axons, where CB1 receptors travel anchored to small transport vesicles13 (Fig. 2B). In these functionally different cell types, the CB1 receptor is invariably found in growth cones (Fig. 2C–F), compartments that allow axons to navigate and establish connections with other neurons. Figure 2C shows a reconstruction of an excitatory growth cone from serial sections solely based on CB1 receptor immunoreactivity, which shows the number and the precise positioning of these receptors. A similar arrangement is observed in GABAergic growth cones (Fig. 2E). Importantly, within the growth cone CB1 receptor is specifically localized to filopodial tips, which effectively function as antennae to sense extracellular signals.10 This suggests that CB1 receptors may mediate extracellular signaling and may allow the growth cones to make navigational decisions (reviewed in Refs.1,5,14). This idea can be probed and tested in vitro by exposing growth cones to endocannabinoids, at which point cannabinoid receptors are removed from the tips of the growth cones, concentrate in the central growth cone compartment, and induce classical primordial extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) signaling.10 This finding indicates that the receptors are functional and relevant not only to differentiation but also to the functional sensitivity of the growing axons (see also Refs.16–18). Moreover, the result suggests that CB1 receptors might be involved in the process through which neurons select their communication partners, which later will come together to form a mature neuronal network.
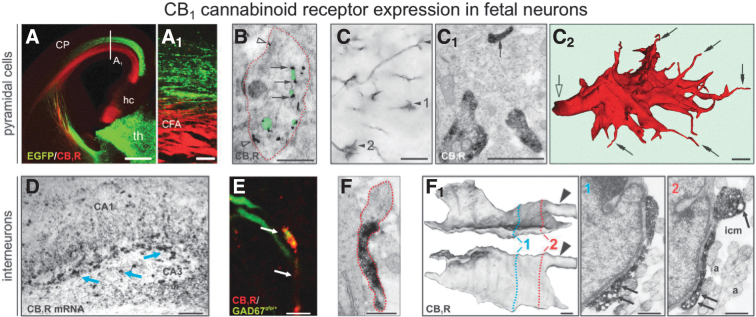
FIG. 2.
Ubiquitous axonal localization of CB1 receptors in cortical neurons. (A, A1) Axons of cortical (in red; CFA, corticofugal axon) but not EGFP-expressing thalamic neurons contain CB1 receptors at embryonic day E16.5. (B) Cross section of an axonal shaft (encircled in red) contains numerous CB1 receptors (arrows) in small transport vesicles (in semitransparent green) and on the plasmalemmal surface (open arrowheads). (C–C2) CB1 receptor-positive corticofugal growth cones (arrowheads) in the internal capsule at E13.5. (C1, C2) Ultrastructural analysis of serial sections from a CB1 receptor-positive growth cone reveals receptors in thick profiles and filopodial tips (arrows), particularly upon three-dimensional reconstruction (C2). (D) Hippocampal interneurons (arrows) express high levels of CB1 receptor mRNA at E18.5. (E) At birth, CB1 receptors are seen in GABAergic axons. (F, F1) Three-dimensional reconstruction using reciprocally perpendicular projections of a single growth cone. Numbers indicate the positions of planar images. Arrowheads indicate the truncated axon. Note that the structure contains numerous vesicles (arrows). Scale bars=500 μm (A), 100 μm (A1, D), 6 μm (E), 1 μm (B, C), and 0.5 μm (C1, C2, F, F1). From “Berghuis P, Rajnicek AM, Morozov YM, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216.” Reprinted with permission from AAAS. This figure is also reproduced with permission from Keimpema et al.13
One way to test these ideas is to use a simple extracellular guidance system in which a point source of gradient of a ligand—in this case a cannabinoid receptor agonist—is puffed in a microgradient onto a growing growth cone.10,19 This intervention could produce either of two responses. The growth cone could extend and turn toward the point source of the gradient, in which case we would classify the response as “attractive.” Alternatively, the growth cone could avoid the gradient, extend and turn away, or in extreme cases, could collapse and retract. This is considered a “repulsive” response. When we conducted this experiment using a cannabinoid receptor agonist, what we found was really exciting. As reported in Berghuis et al.,10 under control conditions the growth cone of cortical interneurons extends regardless of the source of the gradient, and growth is linear. And if one applies a known attractive force, such as brain-derived neurotrophic factor,20 the growth cone extends and turns toward the source of the gradient. However, when the cannabinoid agonist WIN 55,212-2 is puffed onto the cone, we observed an avoidance response. And when the same test was done with differentiated growth cones, which are less motile, we found that they lost their structure, collapsed and degenerated, and their axon withdrew. Was this effect due to direct receptor activation? To address this question, we coincidentally applied a selective CB1 receptor antagonist (AM251), which converted the repulsive response into an attractive one, suggesting that CB1 receptor activation directly affects growth cone motility and neuronal network organization.10 These pharmacological experiments were confirmed using genetic tools. When we deleted CB1 receptors selectively from cortical pyramidal neurons,7 their otherwise organized long-range axons became bundled up. They lost their trajectories, stopped and stalled, and did not invade subcortical territories. As a result, cortical connectivity became fragmented. These results suggest that endocannabinoid signaling controls the processes through which axonal connectivity become organized during brain development.
Naturally, no signaling system works in isolation. During brain development, there is a massive amount of coexistent signaling events, which interact with one other. An important question, therefore, is whether CB1 receptor activation might be downstream or upstream of other signaling systems. Our results8 suggest that the CB1 receptor acts as an upstream regulatory element toward classical axon guidance systems, although extensive work from Patrick Doherty's laboratory21 on endocannabinoid–neurotrophin interactions suggests that, conversely, endocannabinoids can also serve as an effector system (see also Ref.22). We found that CB1 receptor activity can affect the arrangement, number, and location of ROBO1 receptors in axonal growth cones. Interestingly, in coexisting and codeveloping oligodendrocytes, which provide guidance cues, particularly 2-AG, to neurons to grow to, CB2 receptors may in turn tune the amount of SLIT ligands. SLIT and ROBO interactions, similarly as 2-AG and CB1 receptor interactions, are repulsive interactions.3 Thereby, this would be a prototypic amplification loop for axonal repulsion by which endocannabinoids can indeed be integrated and find a place in the hierarchy of coexisting developmental cues and guidance systems in the brain of mice, and most likely also humans (see also Ref.5).
In concluding the first part of our talk, we would like to highlight that the molecular arrangement in a developing immature neuron is different from what we see in adults, because CB1 receptors are in growth cones, which later become axon terminals.5,11 Importantly, growth cones are also rich in endocannabinoid-producing enzymes, such as diacylglycerol lipase-α, whereas the stabilized axon is filled up by the endocannabinoid degrading enzyme monoacylglycerol lipase.13 This arrangement is relevant to how THC may affect and in fact derail the development of axonal connectivity, which is the focus of the second part of this lecture.
What does THC exposure do to the developing brain? When we administered THC to pregnant mouse dams at the relatively low dose of 5 mg/kg, what we saw was an axonal phenotype reminiscent of what is seen in CB1 receptor knockout animals, that is a fasciculation phenotype.23 In other words, THC not only changed the number of axons which coexist, but also their trajectories. Of note, the phenotype obtained by administering THC is less robust than the phenotype seen in CB1 receptor knockout mice, likely because THC is a low-efficacy agonist that only partially enters the placenta. However, because the phenotype we observed is similar to what we see with a CB1 receptor antagonist, we would argue that in this context, THC may act by displacing high-efficacy endocannabinoids from CB1 receptors, thereby limiting their ability to shape axonal connectivity in the brain.15,23 In other words, in its capacity as a partial agonist THC appears to act as a functional antagonist by displacing the full agonist 2-AG from CB1 receptors.
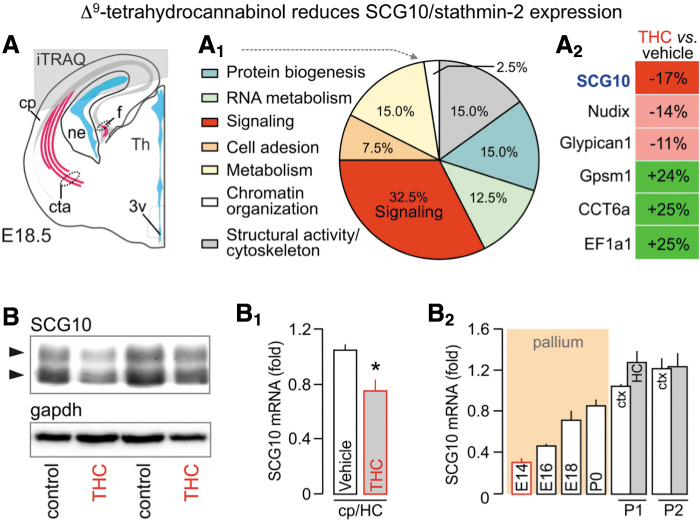
Another relevant question is whether there might be alternative non-cannabinoid receptor targets for THC in the developing brain. We tested this possibility in an experiment in which THC was administered to CB1 receptor knockout mice and their wild-type littermates. We saw that the knockout phenotype was not affected by THC, which is inconsistent with the idea that THC has a secondary target in the fetal brain.23 Furthermore, we used a proteomic approach to gain a more global view of the molecular targets of THC in the developing mouse brain (Fig. 3A). When dams were exposed to THC, we found that only 33–35 proteins changed in the fetal brain, many of which fell within the Gene Ontology (GO) category of signaling systems (Fig. 3A1, A2). One of the affected proteins, SCG10 or neuron-specific stathmin-2, was particularly interesting. Stathmin-2 is only expressed during axonal growth and guidance24 and, therefore, only a subcontingent of cortical neurons expresses it at high levels at any given time. We confirmed the proteomic data by Western blot analysis (Fig. 3B, B1), and further showed that stathmin-2 is expressed throughout the THC dosing period, when cortical connectivity forms in the mouse brain (Fig. 3B2).
FIG. 3.
SCG10 expression in the fetal cerebrum upon THC exposure. (A–A2) The origin of cortical tissues (gray overlay) used for target discovery by mass spectrometry. (A1) Ontology classification of the 35 protein hits based on primary function assignment. Topmost modified protein targets are listed in (A2). SCG10 was significantly downregulated in THC-exposed fetuses. (B–B2) Reduced SCG10 protein (B) and mRNA levels (B1) upon in utero THC exposure (embryonic day 18.5) in the cortical plate (cp) and HC. (B2) Temporal profile of SCG10 mRNA expression during cortical development. Postnatally, the neocortex (ctx) and HC were separated. *p<0.05 THC vs. vehicle. Data were reproduced with permission from Tortoriello et al.23 HC, hippocampus; THC, Δ9-tetrahydrocannabinol.
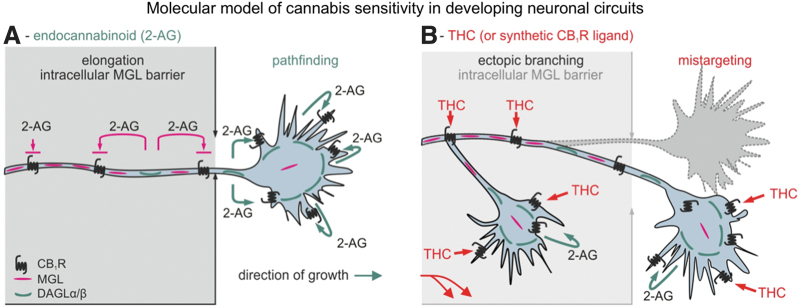
A mouse brain can be a dead end, but through a collaboration with Yasmin Hurd's laboratory, we were able to compare stathmin-2 levels in human fetal brains from cannabis-smoking and control mothers after elective abortion. As reported by Tortoriello et al.,23 there was a significant loss of stathmin-2 at both the mRNA and protein level in fetal brains that had been exposed to cannabis, suggesting that use of the drug during pregnancy downregulates the levels of this particular protein. This may happen because THC interferes with signaling through JNK and/or ERK, which phosphorylate stathmin-2.24 Once phosphorylated, stathmin-2 is destined to degradation.24 A reduction in stathmin-2 levels might have significant consequences because this protein carries a dimer of tubulin molecules, and thereby directly impacts microtubule instability. Indeed, our results suggest that tubulin aging is increased, and thereby neuronal morphology is affected. In a sense, it is as if THC-exposed neurons become frozen in time and are thus unable to correct axonal growth and guidance errors. This point is illustrated in Keimpema et al.,15 as you can see here (Fig. 4A), under physiological conditions, 2-AG is unable to impact cannabinoid receptors, which are trafficked toward the growth cones, because it is immediately degraded by a stable monoacylglycerol lipase contingent and, therefore, CB1 receptors are only activated at physiological positions in the growth cones, allowing for pathfinding decisions to take place. However, THC is not degraded by monoacylglycerol lipase, and in fact, it can cross biological membranes. Therefore, it can affect prematurely CB1 receptor signaling (Fig. 4B), and these signaling events through stathmin-2 signaling and perhaps other signaling cascades induce erroneous turning decisions and collateral formation in neurons, which clearly is detrimental for the assembly and for the precision of how the developing brain forms and how connectivity forms within.
FIG. 4.
Hypothetical model of THC-induced neuronal wiring defects. (A) During axonal development, MGL forms an intracellular enzymatic barrier to prevent 2-AG-driven activation of the CB1 receptor transported along the primordial axon. Thus, we recognize MGL as an enzymatic checkpoint to control 2-AG-dependent morphogenesis and neurite outgrowth. A decrementing MAGL gradient toward the motile growth cone allows 2-AG to activate CB1 receptors, thus impacting growth cone steering decisions. (B) Prenatal exposure to THC (or other cannabinomimetics) can override this endogenous mechanism since these ligands are not substrates of MGL. Thus, THC can prematurely engage CB1 receptors en route to their signaling positions and induce errant second messenger signaling. This can modify neuronal morphology and connectivity. Reproduced from Keimpema et al.15 2-AG, 2-arachidonoyl-glycerol; MGL, monoacylglycerol lipase.
In the last part of this talk, we would like to turn to another important question, namely, the impact of THC on postnatal brain development. We know from the epidemiological literature that early-life exposure to cannabis, especially in the first 15 years, increases the risk of developing psychotic symptoms acutely and later on in life.12,25 This clearly suggests that as the brain matures, also postnatally, it is very much sensitive to cannabis action, and in fact, up until neuronal networks are stabilized and endocannabinoid signaling adopts the classical retrograde messenger functions, any interference by plant-derived cannabinoids may affect how neuronal networks form.
To mimic this, we dosed mice with THC between postnatal days 5 and 16 and found that the treatment is accompanied by a loss of pyramidal neurons and particular types of interneurons in the hippocampus.26 So we can argue that once cells are at a final position or are approaching their final positions, then they might succumb to postnatal THC exposure, particularly if they express CB1 receptors. To explore the molecular basis for this effect we conducted proteomics in THC-treated mice and vehicle-treated controls.26 The analyses were done 2 weeks or 4 months after the end of THC exposure during the period of postnatal days 5–35. Interestingly, even at these late time points, we still find a number of changed proteins, many of which fall into the category of mitochondrial metabolism. This is significant because developing neurons are in the most energy-demanding phase of their life, as they undergo migration and establish their morphologies and connectivity. If their fueling mitochondrial system is disrupted and bioenergetics is impaired, then these cells most likely will die and be eliminated from neuronal networks.
And to test this in vitro, it is particularly possible in a two-color assay. As you can see that even within 1 h, THC exposure will disrupt the mitochondrial membrane potential in a dose-dependent manner in neurons, and once their mitochondrial membrane potential is disrupted, these neurons will ultimately and irrevocably die. And finally, we would show that this indiscriminative effect is really through—and a measure of mitochondrial switch-off or mitochondrial failure is through—a change in membrane integrity in neurons. And what we have done with collaborators, we have built a device that is a nanoindenter,26 which can directly measure the force that biological membranes can withstand. As you can see, again, THC dose-dependently disrupts mitochondrial membrane integrity—in other words, the ability to withstand this pressure force in neurons, at least in vitro. So we do advocate and concur with the idea that during postnatal development, one of the key sites of THC action is the disruption of cellular bioenergetics, most likely through first damaging mitochondria, and then subsequently disorganizing the entire cell, particularly through making its membranes leaky, and thereby destining it to demise.
In conclusion, the results we presented today strongly suggest that the endocannabinoid signaling system has a different cellular organization in immature neurons compared with mature ones. Particularly important is the growth cone domain for these cells. We would advocate that endocannabinoid signaling is detrimental, whether it is too little or too much, and particularly too much can be when using those molecules and those antagonists that inhibit endocannabinoid degradation.8 But most certainly, THC from cannabis affects both pre- and postnatal development, and its effect is really dramatic. It can acutely be through very specific signaling cascades. We have shown one candidate, prenatal candidate, which is the stathmin-2 cascade, whereas postnatally, most likely it directly affects mitochondrial function, and the inability to maintain mitochondrial and membrane integrity.
Abbreviations Used
- 2-AG
2-arachidonoyl-glycerol
- ERK
extracellular signal-regulated kinase
- GO
Gene Ontology
- HC
hippocampus
- ICAL
Impact of Cannabinoids Across the Lifespan
- JNK
c-Jun N-terminal kinase
- MGL
monoacylglycerol lipase
- THC
Δ9-tetrahydrocannabinol
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by the Swedish Research Council (T.Ha.), Novo Nordisk Foundation (T.Ha.), Hjärnfonden (T.Ha.), and the European Research Council (SECRET-CELLS, ERC-2015-AdG-695136; T.Ha.).
Cite this article as: Harkany T, Cinquina V (2021) Physiological rules of endocannabinoid action during fetal and neonatal brain development, Cannabis and Cannabinoid Research 6:5, 381–388, DOI: 10.1089/can.2021.0096.
References
- 1. Silva CG, Peyre E, Nguyen L. Cell migration promotes dynamic cellular interactions to control cerebral cortex morphogenesis. Nat Rev Neurosci. 2019;20:318–329. [DOI] [PubMed] [Google Scholar]
- 2. Llinares-Benadero C, Borrell V. Deconstructing cortical folding: genetic, cellular and mechanical determinants. Nat Rev Neurosci. 2019;20:161–176. [DOI] [PubMed] [Google Scholar]
- 3. Chédotal A. Roles of axon guidance molecules in neuronal wiring in the developing spinal cord. Nat Rev Neurosci. 2019;20:380–396. [DOI] [PubMed] [Google Scholar]
- 4. Tojima T, Hines JH, Henley JR, Kamiguchi H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat Rev Neurosci. 2011;12:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maccarrone M, Guzmán M, Mackie K, et al. . Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci. 2014;15:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berghuis P, Dobszay MB, Wang X, et al. . Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci U S A. 2005;102:19115–19120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mulder J, Aguado T, Keimpema E, et al. . Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci U S A. 2008;105:8760–8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alpár A, Tortoriello G, Calvigioni D, et al. . Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling. Nat Commun. 2014;5:4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goncalves MB, Suetterlin P, Yip P, et al. . A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol Cell Neurosci. 2008;38:526–536. [DOI] [PubMed] [Google Scholar]
- 10. Berghuis P, Rajnicek AM, Morozov YM, et al. . Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. [DOI] [PubMed] [Google Scholar]
- 11. Jutras-Aswad D, DiNieri JA, Harkany T, Hurd YL. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur Arch Psychiatry Clin Neurosci. 2009;259:395–412. [DOI] [PubMed] [Google Scholar]
- 12. Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keimpema E, Barabas K, Morozov YM, et al. . Differential subcellular recruitment of monoacylglycerol lipase generates spatial specificity of 2-arachidonoyl glycerol signaling during axonal pathfinding. J Neurosci. 2010;30:13992–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harkany T, Guzmán M, Galve-Roperh I, et al. . The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. [DOI] [PubMed] [Google Scholar]
- 15. Keimpema E, Mackie K, Harkany T. Molecular model of cannabis sensitivity in developing neuronal circuits. Trends Pharmacol Sci. 2011;32:551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Argaw A, Duff G, Zabouri N, et al. . Concerted action of CB1 cannabinoid receptor and deleted in colorectal cancer in axon guidance. J Neurosci. 2011;31:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cherif H, Argaw A, Cécyre B, et al. . Role of GPR55 during axon growth and target innervation. eNeuro. 2015;2:ENEURO..0011-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duff G, Argaw A, Cecyre B, et al. . Cannabinoid receptor CB2 modulates axon guidance. PLoS One. 2013;8:e70849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuan XB, Jin M, Xu X, et al. . Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat Cell Biol. 2003;5:38–45. [DOI] [PubMed] [Google Scholar]
- 20. Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. [DOI] [PubMed] [Google Scholar]
- 21. Williams EJ, Walsh FS, Doherty P. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J Cell Biol. 2003;160:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keimpema E, Tortoriello G, Alpár A, et al. . Nerve growth factor scales endocannabinoid signaling by regulating monoacylglycerol lipase turnover in developing cholinergic neurons. Proc Natl Acad Sci U S A. 2013;110:1935–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tortoriello G, Morris CV, Alpar A, et al. . Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 2014;33:668–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin JE, Miller BR, Babetto E, et al. . SCG10 is a JNK target in the axonal degeneration pathway. Proc Natl Acad Sci U S A. 2012;109:E3696–E3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Di Forti M, Marconi A, Carra E, et al. . Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry. 2015;2:233–238. [DOI] [PubMed] [Google Scholar]
- 26. Beiersdorf J, Hevesi Z, Calvigioni D, et al. . Adverse effects of Δ9-tetrahydrocannabinol on neuronal bioenergetics during postnatal development. JCI Insight. 2020;5:e135418. [DOI] [PMC free article] [PubMed] [Google Scholar]