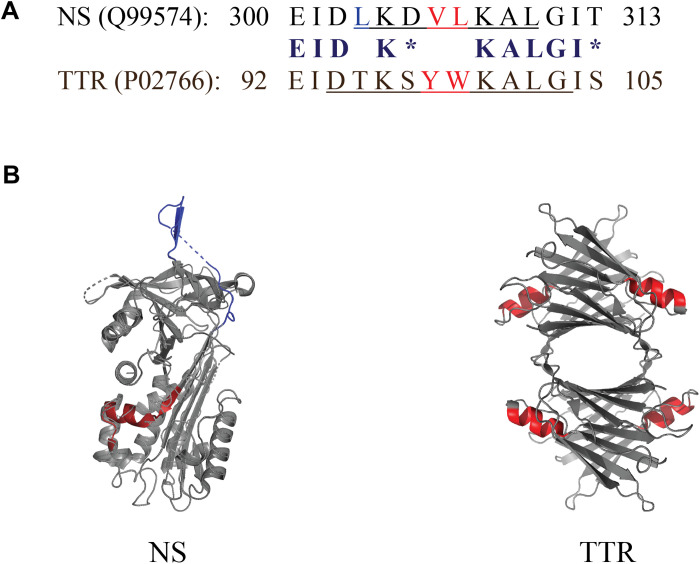
Fig. 4. Short conserved region shared between NS and TTR.
(A) Comparison of the sequences of NS and TTR corresponding to the conserved 14-mer region. Identical residues are indicated in bold, chemically similar residues (blosum62 score equal to or greater than 0) are indicated by asterisks, and hydrophobic residues are shown in red. The specific residues corresponding to an α helix are underlined. Residue numbering includes the N-terminal signal peptides in both cases (16 residues for NS and 20 residues for TTR). (B) PyMOL-generated structures of NS and TTR indicating the position of the 14-residue homologous region (shown in red), which incorporates an α helix in both NS and TTR (four α helices in TTR, one per identical monomeric subunit). The PyMOL rendering of NS has two small regions (indicated by dashed lines) for which the structure was not resolved; the NS RCL is shown in dark blue at the top of the image.