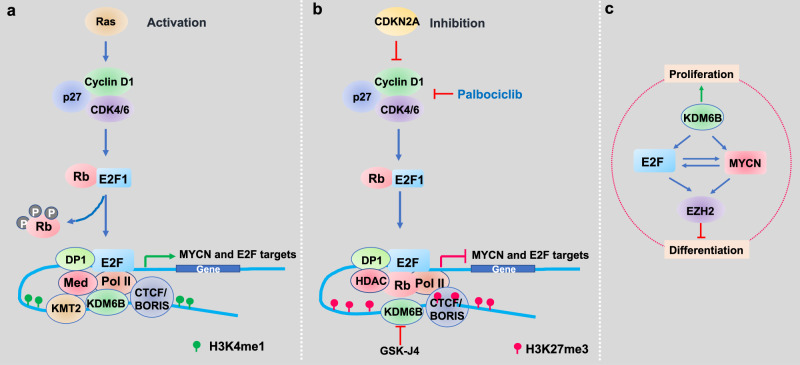
Fig. 8. Working model of KDM6B inhibition in neuroblastoma.
a Upon stimulation by mitogens, KDM6B is recruited to chromatin to maintain the low levels of H3K27me3 at the distal regulatory enhancer regions marked by H3K4me1 overlapping with the CTCF-/BORIS-binding sites, which loops and physically interacts with E2F that binds at the promoter of target genes, together with associated transcriptional machinery including RNA polymerase II and mediators, driving the MYCN and E2F transcriptome. b When inhibited by CDK4/6 inhibitor, the inhibitory pRB complexes with HDAC to suppress gene transcription. When KDM6B is inhibited by GSK-J4, the H3K27me3 will accumulate at the distal regions to displace the H3K4me1 modifier KMT2 and evicts the transcription activators of promoter–enhancer, leading to the downregulation of MYCN and E2F transcriptome. c A network composed by MYCN, E2F, EZH2, and KDM6B regulates the cell proliferation and differentiation of neuroblastoma.