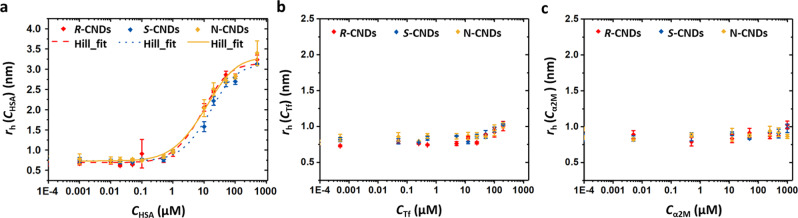
Fig. 2. Change of hydrodynamic radius rh of CNDs (batch #1) as recorded in phosphate-buffered saline (PBS) in dependence of the protein concentration.
a HSA; b Tf; c α2M. From the plots the fit parameters KD, Nmax, n, rh,0, and Δrh,max were obtained, which are listed in Table 2. Kd is the apparent dissociation constant of the CND-protein complex, Nmax is the maximum number of bound proteins per CND under saturation conditions, n is the Hill coefficient, rh,0 is the hydrodynamic radius of the CNDs without attached proteins, and Δrh,max is the difference in effective hydrodynamic radius between CNDs saturated with proteins and CNDs without attached proteins. The shown data were obtained with batch #1. Results are shown as mean values with error bars (i.e. the corresponding standard deviations) from three independent samples (n = 3) over three independent experiments.