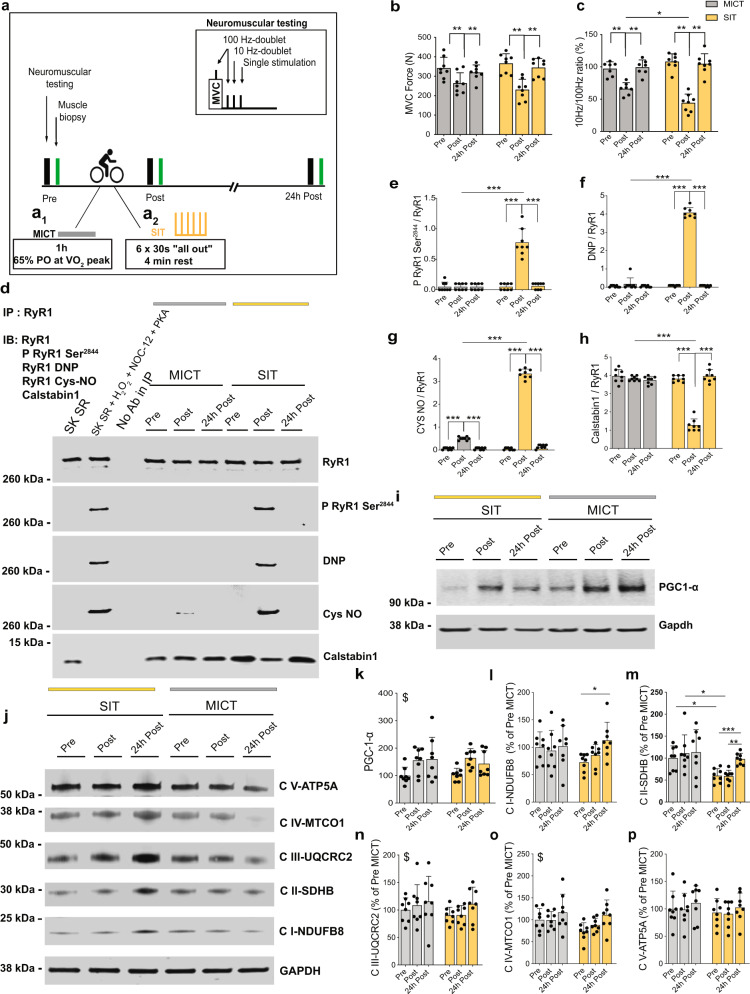
Fig. 1. One session of SIT induces RyR1 post-translational modifications, calstabin1 dissociation from the RyR1 complex and leads to increased OXPHOS protein expression in human muscle.
a Models of MICT (a1) and SIT (a2) in humans. b, c Assessment of b knee extensor maximal voluntary contraction (MVC) force; n = 8 participants per group and c the ratio of electrically evoked forces at 10 and 100 Hz; n = 7 and 8 participants for MICT and SIT, respectively. Two-way ANOVA followed by Sidak’s multiple comparisons test. d Representative immunoblots (IB) of immunoprecipitated (IP) RyR1, RyR1 post-translational modifications and calstabin1 dissociation. DNP (2,4-dinitrophenylhydrazone): RyR1 oxidation. P RyR1 Ser2844: RyR1 phosphorylation at serine 2844. Cys NO: RyR1 nitrosylation. SK SR: skeletal muscle sarcoplasmic reticulum vesicle. SK SR treated with 200 µM H2O2, 250 µM NOC-12 and 5 units PKA per reaction: positive control for RyR1 oxidation and nitrosylation and calstabin1 dissociation. No antibody in IP: negative control. The whole gel and an additional control are shown in Supplementary Fig. 1f. e–h Quantification of immunoblots in (d); n = 8 participants per group. Two-way ANOVA followed by Sidak’s multiple comparisons test. i Representative immunoblots of PGC-1α. j Representative immunoblots of mitochondrial OXPHOS proteins. All the cropped parts of OXPHOS proteins are part of the same blot that is shown in Supplementary Fig. 5. k Quantification of PGC-1α proteins in (i) related to GAPDH protein and expressed as % of Pre-MICT; n = 8 participants per group. Two-way ANOVA. l–p Quantification of OXPHOS proteins in (j) related to GAPDH protein and expressed as % of Pre-MICT; n = 8 participants per group. Two-way ANOVA, followed by Sidak’s multiple comparisons test (l, m). Data are mean ± SD. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and $main effect of time. Source data are provided as a Source Data file.