Figure 5: GLED antinociception in GP120 neuropathic pain model requires μ- and δ-opioid receptors.
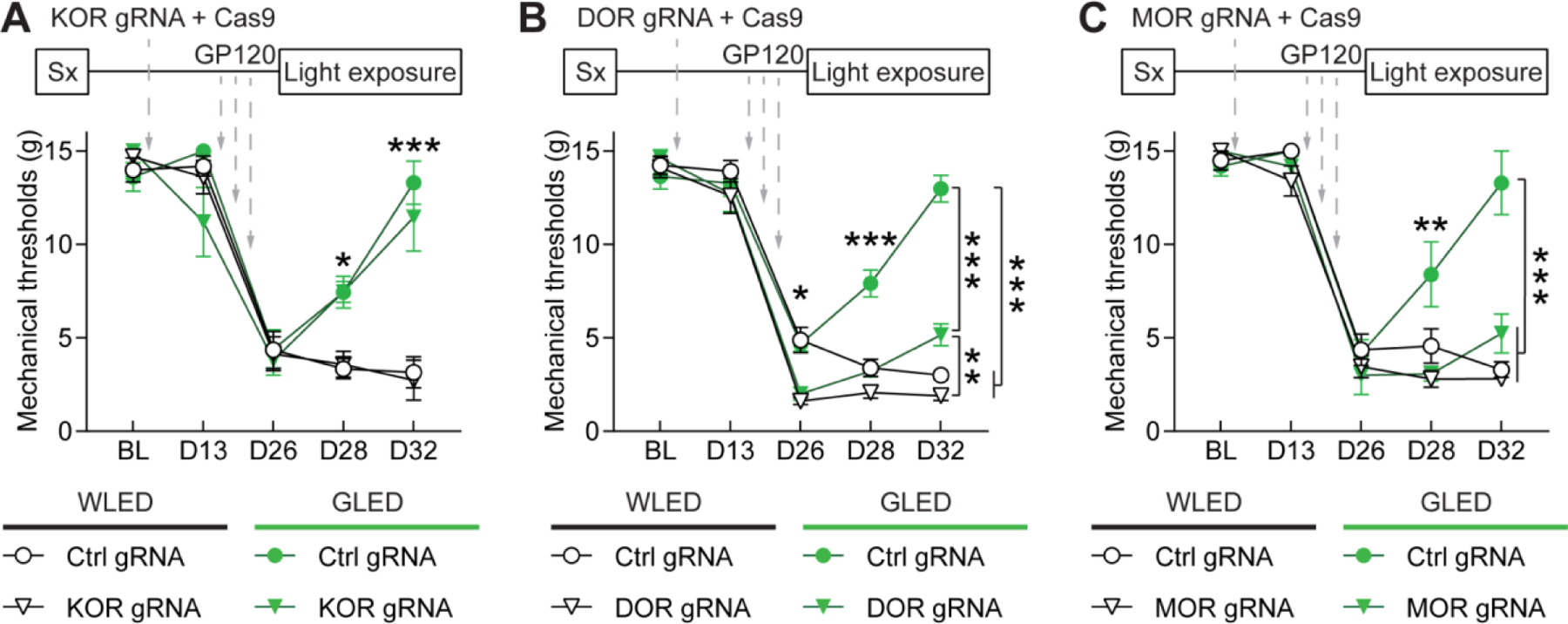
Rats underwent intrathecal (IT) catheter surgery (Sx), and baseline mechanical sensitivity thresholds (BL) were measured two weeks after surgery using Von Frey Filaments. After baseline testing, rats were injected intrathecally with plasmids encoding control, μ- (MOR), δ- (DOR) or κ-opioid receptor (KOR) gRNAs, and the CRISPR-associated endonuclease 9 (Cas9). Mechanical sensitivity was measured two weeks after gene editing, and rats received three IT injections of GP120 (3×300 ng) over a week. One week after the last injection (at D26), hypersensitivity was evaluated to confirm establishment of neuropathic pain. Then rats were then exposed to WLED or GLED for 6 days (8h/day, 100 Lux), and mechanical thresholds were measured at days 28 and 32. (A) Mechanical withdrawal thresholds in rats injected with control or KOR gRNA, before and after exposure to WLED or GLED. KOR editing did not alter GLED antinociceptive effect (n=6, parametric two-way ANOVA followed by Tukey’s posthoc test, *p<0.05, ***p<0.001). (B) Mechanical withdrawal thresholds in rats injected with control or DOR gRNA, before and after exposure to WLED or GLED. DOR editing significantly reduced GLED antinociceptive effect in GP120 rats but did not completely block antinociception compared to WLED rats (n=7–8, parametric two-way ANOVA followed by Tukey’s posthoc test, *p<0.05, **p<0.01, ***p<0.001). (C) Mechanical withdrawal thresholds in rats injected with control or MOR gRNA, before and after exposure to WLED or GLED. MOR editing blocked GLED antinociceptive effect (n=6, parametric two-way ANOVA followed by Tukey’s posthoc test, **p<0.01, ***p<0.001).
