Figure 1-. Interplay of fungal pathogens and host cell lipid microdomains.

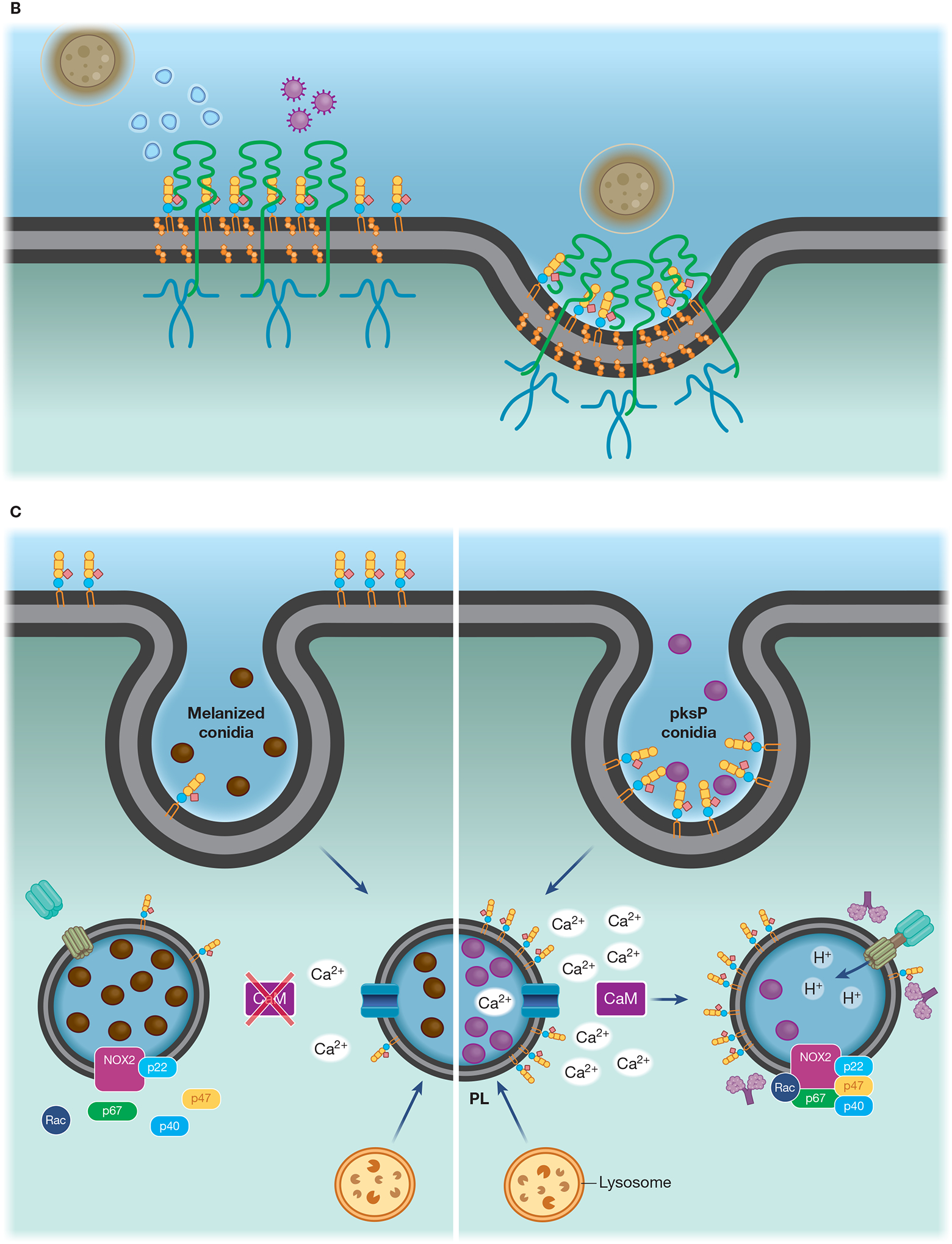
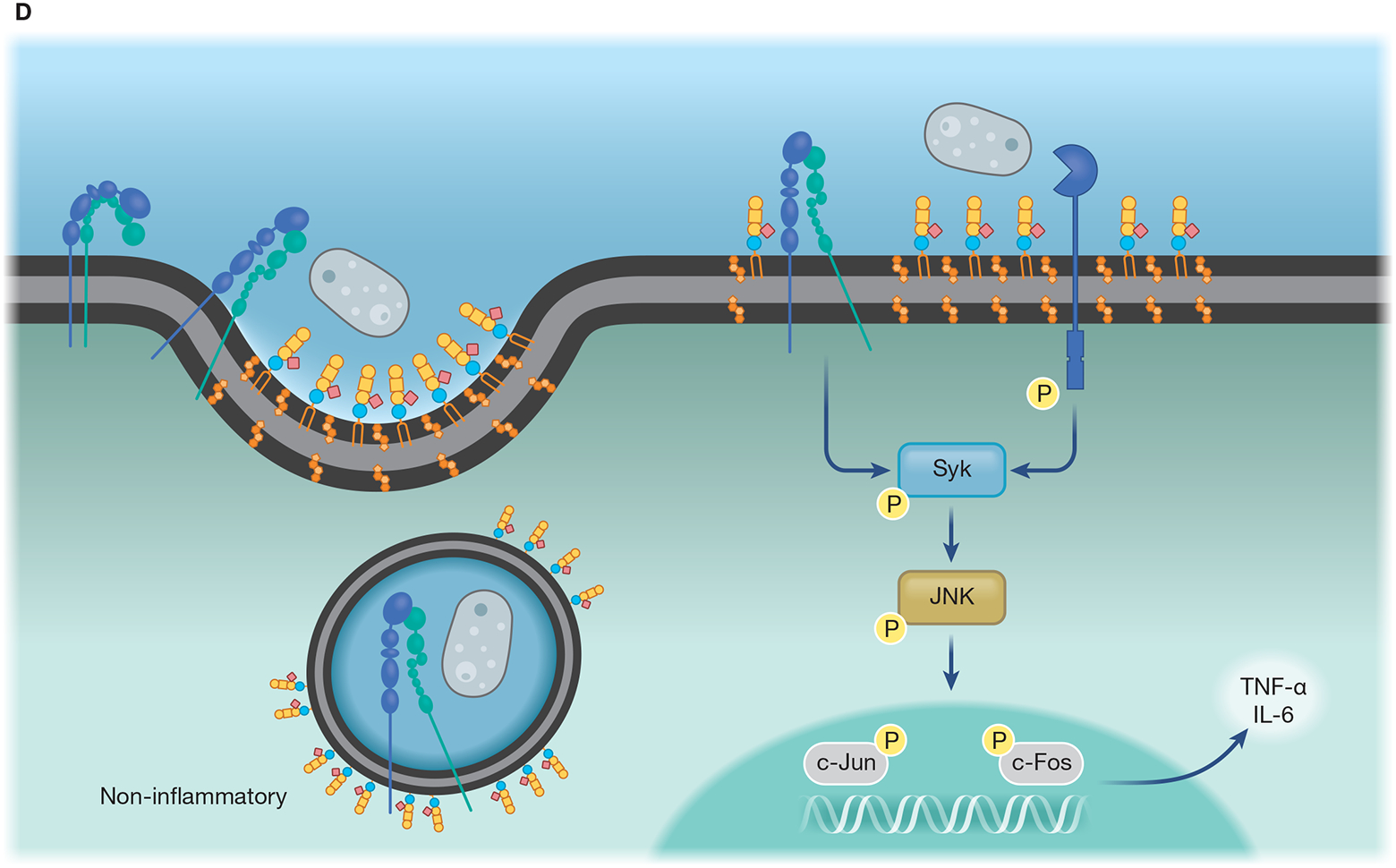
(A) Lipid microdomains enriched in LacCer are binding sites for fungal β-glucan and a signaling platform for CR3 recruitment, allowing this receptor to respond to non-opsonized pathogenic fungi. LacCer domains trigger a signaling pathway through Lyn, PI3K, p38, and PKC, leading to actin polymerization, required for migration, phagocytosis, and ROS production. (B) Caveolae is involved in the invasion of C. neoformans yeast in HBMEC. CD44 is located in caveolin-1-enriched membranes (Cav-1), where it recognizes the fungal hyaluronic acid. The intimate contact between Cav-1 and actin promotes the fungal internalization by HBMEC. The activation of Rho GTPase is correlated with actin remodeling and increased fungal internalization, which could be mediated by Cav-1. In addition, yeast extracellular vesicles and the HIV gp41 stimulate the clustering of CD44 on Cav-1 domain, increasing binding and invasion of C. neoformans yeast. (C) Flotillin domains on phagolysosomal membrane of macrophages are affected by melanin from A. fumigatus conidia. In comparison to non-melanized conidia (pksP), WT conidia block the release of calcium ions from the phagolysosome lumen to the cytoplasm, avoiding the activation of calmodulin (CaM). Conidia melanin impairs the formation of flotillin domains on phagolysosomal membranes and reduces the recruitment and assembly of V-ATPase and NADPH. (D) Recruitment of CR3, the main macrophage receptor for H. capsulatum, to lipid microdomains requires GM1. When β-(1,3)-glucans are exposed on the H. capsulatum cell wall the lipid microdomains allow the cooperation between CR3 and dectin-1, enhancing the inflammatory signaling through Syk, JNK, AP-1 (c-Jun/c-Fos).
