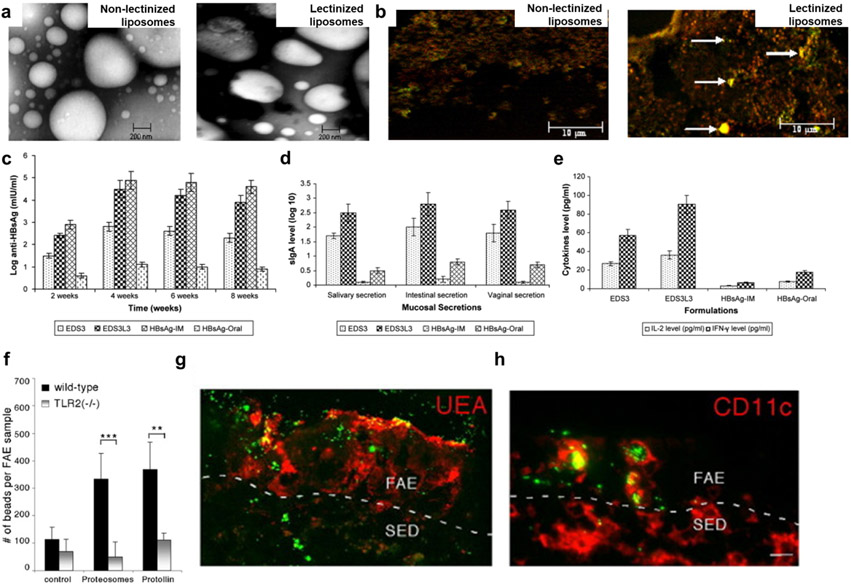
Figure 9. Oral nanomedicine for vaccine applications.
a, TEM images of non-lectinized liposomes and lectinized liposomes. b, In vivo M cell-targeting ability of lectinized liposomes (shown by arrows) c-e, In vivo protective immunity induced by orally administered liposomes carrying hepatitis B surface antigen as measured by serum antibody levels (c), sIgA levels in mucosal secretion (d), and cytokine (IL-2 and IFN-γ) levels in mouse spleen homogenates (e). f-g, In vivo enhancement of TLR2-mediated transepithelial transport of orally administered proteosomes, as measured by counting the number of microspheres in FAE (f) and immunofluorescence images with microspheres (green) associated with M cells (red; g) and intraepithelial CD11c+ DCs (red; h) in the FAE of Peyer's patch. Panels a-e and f-g are reproduced with permission from [250] and [254], respectively.