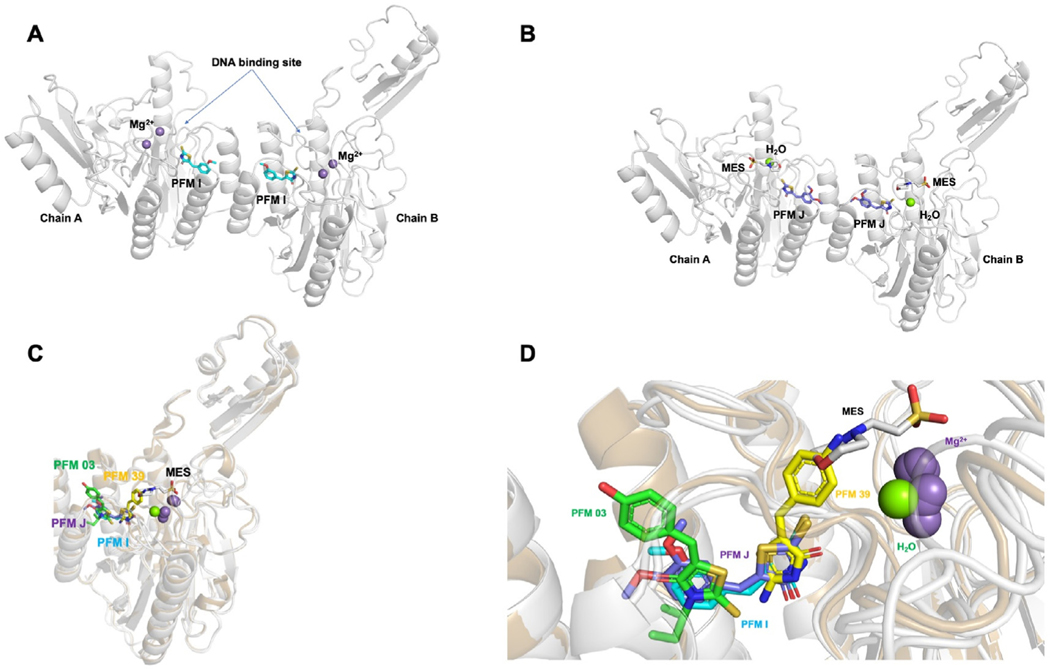
Fig. 3. TmMRE11 crystal structures.
Crystal structures of TmMRE11-PFMI, TmMRE11-PFMJ, and their superposition with other MRE11 inhibitor structures. Panels (A) and (B) depict, respectively, metal ions, buffer and water from crystallization conditions illustrating separately, variations in active site ligands (inhibitors and crystallization reagents) in different crystals structures determined; panels (C) and (D) depict metals, water, buffer and inhibitors all together to illustrate their relative juxtapositions. (A) TmMRE11 dimer in complex with small molecule modulator PFMI (PDB ID 6X1Y). One PFMI bound to each MRE11 subunit, with the rhodanine ring aimed toward the metal active site and methoxyphenyl moiety aimed toward dimerization site. The site of DNA binding has been indicated. (B) TmMRE11 dimer in complex with small molecule modulator PFMJ (PDB ID 6X1Z). One PFMJ bound to each MRE11 subunit, with the rhodanine ring aimed toward the active site, one molecule of MES (2-(N-morpholino)ethanesulfonic acid) buffer coordinated and with methoxy-phenyl moiety aimed toward the dimerization site. (C) Superposition of both complexes TmMRE11-PFMI and TmMRE11-PFMJ in comparison with complex TmMRE11-PFM03 (endo-inhibitor, PDB ID 4O43) and TmMRE11-PFM39 (exo-inhibitor, PDB ID 4O5G), revealing the different binding of the three types of small molecules. The compounds are color coded: PFM03-green; PFMJ-purple; PFMI-cyan; and PFM39-yellow (D) Detail of the superpositions described in (C) in a similar view, but with a slight rotation for a better view, where only one TmMRE11 subunit is visualized to maximize the description of superposed molecular moieties aimed to generate a preliminary pharmacophore.