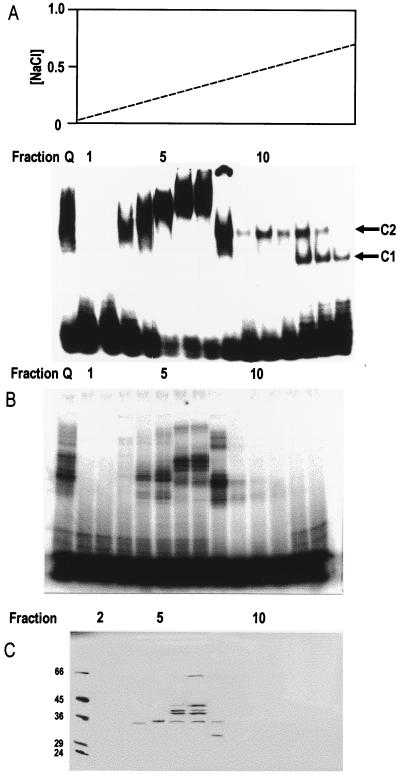
FIG. 3.
Purification by chromatography on a Mono-S column of the protein(s) responsible for complex 2. (A) The upper panel shows a salt gradient eluting the Mono-S column. The lower panel shows EMSA using 20 fmol of the 75-nt probe per assay. Pooled fractions from Mono-Q chromatography after dialysis and Mono-S fractions 1 to 14 are shown. Complex 1 (C1) and complex 2 (C2) are indicated. (B) Phosphorimage of SDS-PAGE of proteins UV cross-linked to the radiolabeled 75-nt probe (following digestion of unprotected probe with RNase T1) in the pooled Mono-Q fractions after dialysis and in Mono-S fractions 1 to 13. (C) SDS-PAGE (silver stained) of proteins of Mono-S fractions 2 to 13 absorbed with poly (U)-Sepharose 4B beads. The beads were washed, and proteins were eluted in SDS sample buffer for electrophoresis. Similar results were seen in two independent experiments.